��Ŀ����
9����Na+�����ʵ���Ũ��Ϊ0.5mol•L-1��ij������Һ�У������ܺ����������ӣ�K+��Mg2+��Ba2+��NO3-��CO32-��SO42-��ȡ100mL����Һ��������ʵ�飨��������ڱ�״���²ⶨ����I�������Һ�м�������ϡ���ᣬ�ڱ�״���·ų�0.56L���壨�����������ܽ⣩��
II����I�����õ���Һ�еμ�����BaCl2��Һ������ɫ����2.33g��
����˵����ȷ���ǣ�������
| A�� | һ�������ڵ�������Mg2+��Ba2+������ȷ���Ƿ���K+��NO3- | |
| B�� | һ�����ڵ�������CO32-��SO42-��CO32-�����ʵ���Ũ��Ϊ0.25mol•L-1��SO42-�����ʵ���Ũ��Ϊ0.1mol•L-1 | |
| C�� | һ�����ڵ�������CO32-��SO42-��K+������K+Ũ�ȡ�0.2mol•L-1 | |
| D�� | һ�����ڵ�������CO32-��SO42-��K+������K+Ũ��Ϊ0.2mol•L-1 |
���� I�������Һ�м�������ϡ���ᣬ�ڱ�״���·ų�0.56L���壨�����������ܽ⣩��˵��һ����CO32-����һ������Mg2+��Ba2+�����ɵ�����Ϊ������̼���壬���ʵ���n��CO2��=n��CO32-��=$\frac{0.56L}{22.4L/mol}$=0.025mol��
II����I�����õ���Һ�еμ�����BaCl2��Һ������ɫ����2.33g��˵����Һ��һ����SO42-����ɫ����Ϊ���ᱵ�����ʵ���n��BaSO4��=n��SO42-��=$\frac{2.33g}{233g/mol}$=0.01mol��
��Һ��һ�����ڵ�����ΪCO32-��SO42-��Na+��һ��������Mg2+��Ba2+��K+��NO3-���Ը�����Һ�е���غ�����������Һ��c��Na+��=0.5mol/L��c��CO32-��=$\frac{0.025mol}{0.1L}$=0.25mol/L��c��SO42-��=$\frac{0.01mol}{0.1L}$=0.1mol/L����������������ɶ࣬���ж���Һ��һ����K+������������Ӳ����ڣ���Һ�е���غ�Ϊ��c��Na+��+c��K+��=2c��CO32-��+2c��SO42-����c��K+��=0.2mol/L����������������ӣ���c��K+����0.2mol/L���ݴ˷���ѡ���жϣ�
��� �⣺I�������Һ�м�������ϡ���ᣬ�ڱ�״���·ų�0.56L���壨�����������ܽ⣩��˵��һ����CO32-����һ������Mg2+��Ba2+�����ɵ�����Ϊ������̼���壬���ʵ���n��CO2��=n��CO32-��=$\frac{0.56L}{22.4L/mol}$=0.025mol��
II����I�����õ���Һ�еμ�����BaCl2��Һ������ɫ����2.33g��˵����Һ��һ����SO42-����ɫ����Ϊ���ᱵ�����ʵ���n��BaSO4��=n��SO42-��=$\frac{2.33g}{233g/mol}$=0.01mol��
��Һ��һ�����ڵ�����ΪCO32-��SO42-��Na+��һ��������Mg2+��Ba2+��K+��NO3-���Ը�����Һ�е���غ�����������Һ��c��Na+��=0.5mol/L��c��CO32-��=$\frac{0.025mol}{0.1L}$=0.25mol/L��c��SO42-��=$\frac{0.01mol}{0.1L}$=0.1mol/L����������������ɶ࣬���ж���Һ��һ����K+������������Ӳ����ڣ���Һ�е���غ�Ϊ��c��Na+��+c��K+��=2c��CO32-��+2c��SO42-����c��K+��=0.2mol/L����������������ӣ���c��K+����0.2mol/L��
A��������֪��K+һ�����ڣ���A����
B��һ�����ڵ�������CO32-��SO42-��K+����B����
C������������֪һ�����ڵ�������CO32-��SO42-��K+������K+Ũ�ȡ�0.2mol•L-1����C��ȷ��
D������������Ӳ����ڣ���Һ�е���غ�Ϊ��c��Na+��+c��K+��=2c��CO32-��+2c��SO42-����c��K+��=0.2mol/L����������������ӣ���c��K+����0.2mol/L����D����
��ѡC��
���� ���⿼���������ʡ���Ӧ��������ӷ�Ӧ��Ķ�����ϵ���Ѷ��еȣ�ע���������ӷ�Ӧ�����ݵ���غ��ж�K+�Ƿ���ڣ��DZ�����ѵ㡢�״��㣬�Ѷ��еȣ�

| A�� | N2 | B�� | NO2 | C�� | NO | D�� | N2O3 |
| A�� | ���������Ļ�ѧʽΪ��C4H8O2 | B�� | ���Ľṹ��ʽΪ�� | ||
| C�� | ���Ȼ�̼�ĵ���ʽΪ��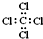 | D�� | ������ļ���ʽΪ�� |
| A�� | AlCl3��Һ���ռ���Һ��Ӧ����n��OH-����n��Al3+��=7��2ʱ��2Al3++7OH-�TAl��OH��3��+AlO2-+2H2O | |
| B�� | CuCl2��Һ��NaHS��Һ��Ӧ����n��CuCl2����n��NaHS��=1��2ʱCu2++2HS-�TCuS��+H2S�� | |
| C�� | Cl2��FeBr2��Һ��Ӧ����n��Cl2����n��FeBr2��=1��1ʱ��2Fe2++4Br-+3Cl2�T2 Fe3++2Br2+6Cl- | |
| D�� | Fe��ϡ���ᷴӦ����n��Fe����n��HNO3��=3��8ʱ��3Fe+2NO3-+8H+�T3Fe2++2NO��+4H2O |
| A�� | ����� | B�� | ���ᱵ | C�� | ���軯�� | D�� | �⻯�� |
| A�� | ��������ƽ��ȡ3.25g NaCl | |
| B�� | ����ʽ�ζ�����ȡ20.00mL KMnO4��Һ | |
| C�� | ����Ͳ��ȡ10.51 mL���� | |
| D�� | ������ƿ����216mL 0.1mol/L NaOH��Һ |
| A�� | �Ҵ���Ũ�����ᷴӦ CH3CH2OH+HBr$\stackrel{��}{��}$CH3CH2Br+H2O | |
| B�� | ������������������Һ���� CH3CH2Br+NaOH$��_{��}^{ˮ}$CH3CH2OH+NaBr | |
| C�� | ��������ͨ�������Ķ�����̼ 2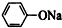 +CO2+H2O��2 +CO2+H2O��2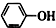 +Na2CO3 +Na2CO3 | |
| D�� | ��ȩ������ 2CH3CHO+O2$��_{��}^{����}$2CH3COOH |
| ���� | ���� | ���� | |
| A | �ٵμ�ϡHNO3 �ڵμ�BaCl2��Һ | ���������� ��ɫ���� | ԭ��Һһ����Ag+ |
| B | �μ�ϡ���� | �д������ݲ��� | ԭ��Һһ����CO32- |
| C | �ٵμ�ϡHCl �ڵμ�AgNO3��Һ | ���������� ��ɫ���� | ԭ��Һһ����Cl- |
| D | �ټ�KSCN��Һ �ڵμ���ˮ | ���������� ��Һ�ʺ�ɫ | ԭ��Һһ����Fe2+ |
| A�� | A | B�� | B | C�� | C | D�� | D |
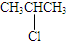 ��
�� ��
�� ��
��