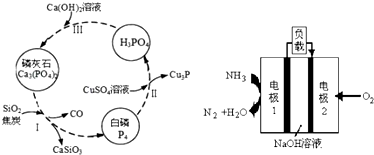
��Ŀ����
10��ij����M��һ����������������Ϊȷ���仯ѧʽ��ijС����Ʋ����������ʵ�飺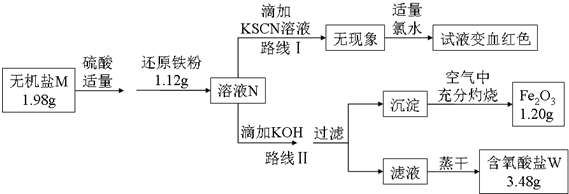
��֪��
������M���ɼ����Ӻ�һ�ֺ��������ɣ�������е�ԭ�Ӹ�����Ϊ2��1��4��
����ͼ�У���1.98g����������ˮ���μ�����ϡ������ټ���1.12g��ԭ���ۣ�ǡ����ȫ��Ӧ�û����ҺN��
�۸�С��ͬѧ����ҺN��Ϊ���ȷݣ��ֱ�·�ߢ�·�ߢ����ʵ�飮
����·�ߢ��У���������ҺN�еμ�����KOH��Ԫ��X�պó�����ȫ�����˺����ڿ����г�����յô�����Fe2O3��ĩ1.20g���ٽ���Һ��һ�����������ɣ�ֻ�õ�3.48g�����IJ����ᾧˮ������W��
�밴Ҫ��ش��������⣺
����·�ߢ�������֪����ҺN�к��е���������Fe2+��
����ʵ������ͼ���Ƶã���������W�Ļ�ѧʽ��K2SO4 ����·�ߢ��֪��1.98g����M��������Ԫ�ص�����Ϊ0.78g��
������M��1.12g��ԭ����ǡ����ȫ��Ӧ������ҺN�Ļ�ѧ��Ӧ����Ϊ2Fe+K2FeO4+4H2SO4�T3FeSO4+K2SO4+4H2O��
���� 1.2g�����������ʵ���Ϊ��$\frac{1.2g}{160g/mol}$=0.0075mol������ҺN�к�����Ԫ�ص����ʵ���Ϊ��0.0075mol��2��2=0.03mol������Ϊ��56g/mol��0.03mol=1.68g��1.12g����������M��һ������FeԪ�أ���ԭ������M����Ԫ�ص����ʵ���Ϊ0.03mol-0.02mol=0.01mol���������EΪNa2SO4��2.84g Na2SO4���ʵ���Ϊ0.02mol��������W�к���Ԫ�أ�����WΪ����������ҷ����е�ԭ�Ӹ�����Ϊ1��2��4���ɵ�M�Ļ�ѧʽΪK2FeO4��
��1��·�ߢ�Ϊ�����������ӵķ�����
��2��K2FeO4��ϡ���ᡢ���۷�Ӧ������������������غ�ˮ�����������غ��֪WΪ����أ�����n=$\frac{m}{M}$�����K2FeO4�����ʵ������ٸ���n=nM����������ӵ�������
��3������K2FeO4��ϡ���ᡢ���۷�Ӧ������������������غ�ˮд����Ӧ�Ļ�ѧ����ʽ��
��� �⣺1.2g�����������ʵ���Ϊ��$\frac{1.2g}{160g/mol}$=0.0075mol������ҺN�к�����Ԫ�ص����ʵ���Ϊ��0.0075mol��2��2=0.03mol������Ϊ��56g/mol��0.03mol=1.68g��1.12g����������M��һ������FeԪ�أ���ԭ������M����Ԫ�ص����ʵ���Ϊ0.03mol-0.02mol=0.01mol���������EΪNa2SO4��2.84g Na2SO4���ʵ���Ϊ0.02mol��������W�к���Ԫ�أ�����WΪ����������ҷ����е�ԭ�Ӹ�����Ϊ1��2��4���ɵ�M�Ļ�ѧʽΪK2FeO4��
��1������·�ߢ��֪��N��Һ��һ�������������ӣ�
�ʴ�Ϊ��Fe2+��
��2��1.2g�����������ʵ���Ϊ��$\frac{1.2g}{160g/mol}$=0.0075mol������Һ�к�����Ԫ�ص����ʵ���Ϊ��0.0075mol��2��2=0.03mol������Ϊ��56g/mol��0.03mol=1.68g��1.12g����������M��һ������FeԪ�أ�����M�����е�ԭ�Ӹ�����Ϊ1��2��4����M�Ļ�ѧʽΪ��Na2FeO4���������̿�֪��Na2FeO4��ϡ���ᡢ��ԭ���۷�Ӧ������������������أ����������غ��֪WΪ����أ�1.98gK2FeO4�����ʵ���Ϊ$\frac{1.98g}{198g/mol}$=0.01mol��0.01molK2FeO4�к���0.02mol�����ӣ����м����ӵ�����Ϊ39g/mol��0.02mol=0.78g��
�ʴ�Ϊ��K2SO4 ��0.78��
��3��1.66gNa2FeO4�����ʵ���Ϊ0.01mol��1.12g��ԭ���۵����ʵ���Ϊ0.02mol������Na2FeO4�뻹ԭ���۰����ʵ���֮��Ϊ1��2��Ӧ�Ļ�ѧ��Ӧ����Ϊ��2Fe+K2FeO4+4H2SO4�T3FeSO4+K2SO4+4H2O��
�ʴ�Ϊ��2Fe+K2FeO4+4H2SO4�T3FeSO4+K2SO4+4H2O��
���� ���⿼����̽��������ɵķ�������Ŀ�Ѷ��еȣ������漰������ɵIJⶨ��Ũ��������ʡ����ӷ���ʽ����ѧ����ʽ����д�����ӵļ��鷽����֪ʶ������֪ʶ��϶࣬�ۺ��Խ�ǿ����ֿ�����ѧ�����Ӧ�û���֪ʶ��������

| A�� | ͬ����Ԫ�غ������������˵���������Ӷ����� | |
| B�� | ��������Ų���ͬ������ѧ����Ҳ��ͬ | |
| C�� | �κ�Ԫ�ص�ԭ�Ӷ����ɺ�����Ӻͺ������ӡ�������� | |
| D�� | ${\;}_{17}^{35}Cl$��${\;}_{17}^{37}Cl$�õ���������ͬ |
| A�� | ����ʽΪC3H8��C6H14�������л���һ����Ϊͬϵ�� | |
| B�� | ͬϵ��Ļ�ѧ�������ƣ�����������̼ԭ�����ĵ������ֹ����Ա仯 | |
| C�� | ��������ͬϵ�����Է���������ֵһ�����14 | |
| D�� | ����������һ����CH2ԭ���ŵĻ�����ض���Ϊͬϵ�� |
| A�� | Ũ�����Ũ�����ڿ����г��ڷ���ʱŨ�Ⱦ����� | |
| B�� | SO2��Cl2����ʹƷ����Һ��ɫ | |
| C�� | ŨH2SO4��ϡH2SO4��п��Ӧʱ���ܲ������� | |
| D�� | H2S��HI�����������ŨH2SO4��ȡ |
| A�� | H2��O2��SO2 | B�� | CO2��H2S��Cl2 | C�� | HCl��HBr��HI | D�� | NH3��HCl��CO2 |
��1��C�ļ۵����Ų�ʽΪ2s22p3��д��һ�ֹ�ҵ�Ʊ�����F�����ӷ���ʽ2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$2OH-+H2��+Cl2����
��2��B��D��E��ɵ�һ�����У�E����������Ϊ43%��������Ϊ�������������ˮ��Һ��ͨ������F���ʷ�Ӧ�Ļ�ѧ����ʽΪ2Na2CO3+Cl2+H2O�TNaCl+NaClO+2NaHCO3��
��3������ЩԪ����ɵ����ʣ�����ɺͽṹ��Ϣ�����
| ���� | ��ɺͽṹ��Ϣ |
| a | ���зǼ��Թ��ۼ��Ķ�Ԫ���ӻ������ԭ����֮��Ϊ1��1 |
| b | ��ѧ���ΪBDF2 |
 ������Bԭ�ӵ��ӻ���ʽΪsp2��
������Bԭ�ӵ��ӻ���ʽΪsp2��