��Ŀ����
8����ѧ�ҷ���C60���ӣ�ͼ1����60��̼ԭ�ӹ��ɣ�������״������ͼ1C��������C=C��������ֽ�����ϩ��1991���ѧ���ַ���һ��̼�ĵ���--̼���ܣ����������ε�̼ԭ�ӹ��ɵľ��кܴ�������״����ӣ�ͼ1D����ͼ1A��ͼ1B�ֱ��ǽ��ʯ��ʯī�Ľṹʾ��ͼ��ͼ��С�ڵ��С��Ȧ������̼ԭ�ӣ���1�����ʯ��ʯī������ϩ��̼�����������ʻ���Ϊͬ�������壬���������������ϴ��ڽϴ�IJ��죬��ԭ����̼ԭ�����з�ʽ��ͬ��
��2��ͬ�����£�����ϩ��ʯī�ֱ�����嵥��F2��Ӧʱ������ϩ�Ļ�ѧ���ʱ�ʯīҪ���ã��������ǣ�����ϩ����C=C�����ӳɣ�
��3��ȼ������֮������δ������ƹ㣬���Ͼ��õ����ⷽ����δ��ȫ����⣬������H2���������⣬��������̼�������п��ܳ�Ϊ������ϵ��ǣ�̼���ܣ�
��4��ͼ2�Ǵ�NaCl��CsCl����ṹͼ�зָ�����IJ��ֽṹͼ�����ж�NaCl����ṹ��ͼ���ǣ�2����3�� ������ţ���

��5��ͼ1A���ʯ�ṹ��һ��̼ԭ�ӱ�6 �������֣�����Ԫ�����ã�
��6����ͼ1B��ͬһ���е�ԭ�ӹ������������������Σ��˵��������ڵļص�������ã��γ�ij����ͭɫ�����ʣ����е�Ԫ�ؼ��á���ʾ����ԭ�ӷֲ���ͼ3��ʾ�������ʵĻ�ѧʽΪKC8 ��
���� ��1�����ʯ��ʯī������ϩ��̼��������������̼ԭ���ڿռ���Ų���ʽ��ͬ���������ʽṹ�����������ʴ��⣻
��2������ϩ�к���̼̼˫�������Ա�ʯī���ã�������F2�����ӳɷ�Ӧ��
��3���������ϸ����ʵĿռ�ṹ��֪������̼���ܱ������������H2������������ϣ�
��4���Ȼ�����6��Na����Cl���γɵ����������м��Χһ��Cl����Na�����侧����һ�������壬�ݴ˴��⣻
��5�����ʯ����״�ṹ�У�ÿ��̼ԭ�����γ�4�����ۼ�������ͼ1A���ʯ�ṹ��֪��һ��̼ԭ�ӱ�6����Ԫ�����ã�
��6����ͼ��ʾ��Kԭ����Ƕ���������ε����ģ�������������Χ��6��δ��ǶKԭ�ӵ�Cԭ���������νṹ����ÿ����������Χ����6��Cԭ�ӣ���̼ԭ��Ϊ3���������ι��У����ݾ�̯�����㣮
��� �⣺��1�����ʯ��ʯī������ϩ��̼��������������̼ԭ���ڿռ���Ų���ʽ��ͬ���������ʻ��м�����죻
�ʴ�Ϊ��̼ԭ�����з�ʽ��ͬ��
��2������ϩ�к���̼̼˫�������Ա�ʯī���ã�������F2�����ӳɷ�Ӧ��
�ʴ�Ϊ������ϩ����C=C�����ӳɣ�
��3���������ϸ����ʵĿռ�ṹ��֪������̼���ܱ������������H2������������ϣ�
�ʴ�Ϊ��̼���ܣ�
��4���Ȼ�����6��Na����Cl���γɵ����������м��Χһ��Cl����Na�����侧����һ�������壬��ѡ��2����3����
��5�����ʯ����״�ṹ�У�ÿ��̼ԭ�����γ�4�����ۼ�������ͼ1A���ʯ�ṹ��֪��һ��̼ԭ�ӱ�6����Ԫ�����ã��ʴ�Ϊ��6��
��6����ͼ��ʾ��Kԭ����Ƕ���������ε����ģ�������������Χ��6��δ��ǶKԭ�ӵ�Cԭ���������νṹ����ÿ����������Χ����6��Cԭ�ӣ���̼ԭ��Ϊ3���������ι��У��ʽṹ��Kԭ����Cԭ����Ŀ֮��Ϊ1����6+6��$\frac{1}{3}$��=8���ʻ�ѧʽΪKC8��
�ʴ�Ϊ��KC8 ��
���� ���⿼�����ʽṹ���ʣ���̼Ԫ�ص����ֵ��ʵĿռ�ṹͼΪ��������㣬�ۺ������ʽṹ����Ӧ��֪ʶ�㣬�������Ӧ�߱�һ���Ĵ�����Ϣ�Ϳռ�����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��������з���δ�ӷ�ʯ��Ӧ�������ӷ�ʯ | |
| B�� | ��Һ�������Ƚ��´�Һ��ֳ����Ե�Ƭ�̺��ٷֳ��ϲ�Һ�� | |
| C�� | ��ˮ���弴���л��ܼ��к�ˮ����ȡ�� | |
| D�� | ��ȥNaCl����KNO3��������ɱ�����Һ��Ȼ�������ᾧ�����ȹ��� |

| A�� | a��ʹ�������������Һ��ɫ | B�� | b�ķ���ʽΪC5H12 | ||
| C�� | b��c��Ϊͬϵ�� | D�� | dΪƽ���η��ӣ��������� |
| A�� | ${\;}_{92}^{235}$U��������Ϊ235 | |
| B�� | ${\;}_{92}^{235}$U��${\;}_{92}^{238}$U�����ֺ��أ����ǻ���Ϊͬ�������� | |
| C�� | �˷�Ӧ���ڻ�ѧ�仯 | |
| D�� | �������ú��ܷ��ϡ���̼���á�Ҫ�� |
| A�� | ���ȿ�������ʳƷ��װ��Ҳ�������ڽ������� | |
| B�� | �������ȿ�������ұ������Ҳ�������ͻ���� | |
| C�� | ���������ȿ�����������θ����࣬Ҳ�������Ʊ�һЩ���� | |
| D�� | ��������������ˮ��Ҳ������������ |
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | 0.1mol/LCH3COONa��0.1mol/LHCl��Һ�������ϣ�c��Na+��=c��Cl-����cCH3COO-����c��OH-�� | |
| B�� | 0.1mol/LNH4Cl��0.1mol/L��ˮ�������ϣ�pH��7����c��NH3•H2O����c��NH4+����c��Cl-����c��OH-�� | |
| C�� | 0.1mol/LNa2CO3��0.1mol/L NaHCO3��Һ�������ϣ�3��Na+��=2[c��CO32-��+c��HCO3-��+c��H2CO3��] | |
| D�� | 0.1mol/LNa2C2O4��0.1mol/LHCl��Һ�������ϣ�H2C2O4Ϊ��Ԫ���ᣩ��2c��C2O42-��+c��HC2O4-��+c��OH-��=c��Na+��+c��H+�� |
| A�� | NaHCO3�TNa++H++CO32- | B�� | NaHSO4�TNa++H++SO42- | ||
| C�� | MgCl2�TMg2++2Cl- | D�� | Ca��OH��2�TCa2++2OH- |
| ��� | A | B | C |
�� �� װ �� | 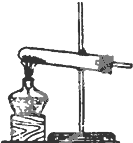 |  | 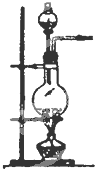 |
��2��ʵ������ʳ�ι����Ũ�������Ȼ�������ʱ����ѡ�õķ���װ����C��д��ţ���д����ʱ�ķ�Ӧ����ʽNaCl+H2SO4 $\frac{\underline{\;��\;}}{\;}$NaHSO4+HCl����
��3��������غͶ������̶���ǿ�����������ɽ�Ũ��������Ϊ������
��a�� ��Ũ�����������������ķ�Ӧ����ʽ���£�
2KMnO4+16HCl��2KCl+2MnCl2+5Cl2��+8H2O
���á������š�����������ʽ�ϱ������ת�Ƶķ������Ŀ��
�ڷ�Ӧ��ClԪ�ر�������KMnO4��������������1mol����ת��ʱ������������11.2������״̬�£���
��b�� ��Ũ����Ͷ���������������ʵ��װ����ͼ��ʾ��
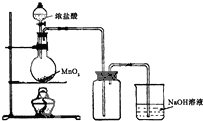
��д��Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽ_MnO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+Cl2��+2H2O
��ʵ�������ϴ����ʱ��Ϊ�˼�����ƿ�в��������Ի�������Ⱦ����������ƿ�м������Һ��NaOH���йصĻ�ѧ����ʽCl2+2NaOH=NaCl+NaClO+H2O��