��Ŀ����
��һ���ݻ��̶�Ϊ1L���ܱ������У�������ӦmA��g��+nB��g���TpC��g����H=������Ӧ�������ͼ��ʾ������ϸ�������ݱ������ݣ��ش��������⣺
��1��m=______��n=______��p=______��
��2������C��ƽ����Ӧ����
��0min����2min��v��C��=______��
��2min����4min��v��C��=______��
��3���ɱ������ݿ�֪��Ӧ�ڵ�4min����6minʱ����ƽ��״̬�����ڵ�2min����6min����8minʱ�ֱ�ı���ijһ��Ӧ��������ı�������ֱ�����ǣ�
�ٵ�2min______��______���ڵ�6min______���۵�8min______��
��4������÷�Ӧ����ƽ��״̬���¶��µ�ƽ�ⳣ��______��
��5�����ӿ�ʼ����4min����ƽ��ʱ��Ӧ�ų�������Ϊ235.92kJ����÷�Ӧ�ġ�H=______��
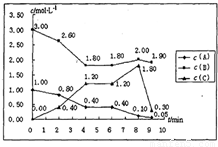
���𰸡���������1���������ʵ���Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȼ���m��n��p��ֵ��
��2������v= ����v��C����
����v��C����
��3��Ӱ�컯ѧ��Ӧ���ʵ������У�ʹ�ô������ı��¶ȡ�Ũ�ȡ��ı����ʵı�����ȣ�
��4��ƽ�ⳣ��Ϊ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ������k= ������ƽ��ʱŨ�ȼ��㣻
������ƽ��ʱŨ�ȼ��㣻
��5���ڿ�ʼ����4min����ƽ��ʱ��A���ʵ�������Ϊ0.6mol/L×1L=0.6mol�����ݻ�ѧ����ʽ���㼴�ɣ�
����⣺��1����ǰ4min�ڣ�A��B��C��Ũ�ȵı仯��֮�ȵ��ڻ�ѧ������֮�ȣ�����m��n��p=��1-0.4������3-1.8������1.2-0��=1��2��2������m=1��n=2��p=2��
�ʴ�Ϊ��1��2��2��
��2����0min����2min��v��C��= =0.2mol/��L?min������2min����4min��v��C��=
=0.2mol/��L?min������2min����4min��v��C��= =0.4mol/��L?min����
=0.4mol/��L?min����
�ʴ�Ϊ��0.2mol/��L?min����0.4mol/��L?min����
��3�����ڵ�0��2min�ڣ�A��Ũ�ȼ�С��0.2mol/l����2��4min��A��Ũ����ԭ�����ϼ�С��0.4mol/l����ѧ��Ӧ���ʼӿ��ˣ�������ʹ�ô����������¶ȣ�
�ʴ�Ϊ��ʹ�ô����������¶ȣ�
�ڵ�6min ����8min����Ϊ��Ӧ�Ũ��Ӧ���Ǽ�С�����ƣ�����B��Ũ�ȴ�1.8mol/l���ӵ���2.0mol/l�����Կ�֪һ���Ǽ�����B���ʣ�
�ʴ�Ϊ������B��Ũ�ȣ�
�۵�8min����9minʱ����ڣ�C��Ϊ���������Ũ��Ӧ����������ƣ��������ݱ�������Ũ�ȼ�С�ˣ�һ���Ǽ�����C��Ũ�ȣ�
�ʴ�Ϊ����СC��Ũ�ȣ�
��4��4minʱ����Ӧ��ƽ�⣬��ʱc��A��=0.4mol/L��c��B��=1.8mol/L��c��C��=1.2mol/L��
����ƽ�ⳣ��k= =
= =
= mol-1?L��
mol-1?L��
�ʴ�Ϊ�� mol-1?L��
mol-1?L��
��5����4�������Ȼ�ѧ����ʽ���裺��Ӧ���ʱ�ֵΪX��
A��g��+2B��g�� 2C��g����H=-XkJ/mol
2C��g����H=-XkJ/mol
1mol XkJ
��1mol/l-0.4mol/l��×1L=0.6mol 235.92kJ
���� �����X=393.2��
�����X=393.2��
���Է�Ӧ�ȡ�H=-393.2kJ/mol��
�ʴ�Ϊ��-393.2kJ/mol��
���������⿼����ѧ��Ӱ�컯ѧ��Ӧ���ʵ����غ��йط�Ӧ���ʵļ��㣬��һ�����ۺ��ԣ�
��2������v=
 ����v��C����
����v��C������3��Ӱ�컯ѧ��Ӧ���ʵ������У�ʹ�ô������ı��¶ȡ�Ũ�ȡ��ı����ʵı�����ȣ�
��4��ƽ�ⳣ��Ϊ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ������k=
 ������ƽ��ʱŨ�ȼ��㣻
������ƽ��ʱŨ�ȼ��㣻��5���ڿ�ʼ����4min����ƽ��ʱ��A���ʵ�������Ϊ0.6mol/L×1L=0.6mol�����ݻ�ѧ����ʽ���㼴�ɣ�
����⣺��1����ǰ4min�ڣ�A��B��C��Ũ�ȵı仯��֮�ȵ��ڻ�ѧ������֮�ȣ�����m��n��p=��1-0.4������3-1.8������1.2-0��=1��2��2������m=1��n=2��p=2��
�ʴ�Ϊ��1��2��2��
��2����0min����2min��v��C��=
 =0.2mol/��L?min������2min����4min��v��C��=
=0.2mol/��L?min������2min����4min��v��C��= =0.4mol/��L?min����
=0.4mol/��L?min�����ʴ�Ϊ��0.2mol/��L?min����0.4mol/��L?min����
��3�����ڵ�0��2min�ڣ�A��Ũ�ȼ�С��0.2mol/l����2��4min��A��Ũ����ԭ�����ϼ�С��0.4mol/l����ѧ��Ӧ���ʼӿ��ˣ�������ʹ�ô����������¶ȣ�
�ʴ�Ϊ��ʹ�ô����������¶ȣ�
�ڵ�6min ����8min����Ϊ��Ӧ�Ũ��Ӧ���Ǽ�С�����ƣ�����B��Ũ�ȴ�1.8mol/l���ӵ���2.0mol/l�����Կ�֪һ���Ǽ�����B���ʣ�
�ʴ�Ϊ������B��Ũ�ȣ�
�۵�8min����9minʱ����ڣ�C��Ϊ���������Ũ��Ӧ����������ƣ��������ݱ�������Ũ�ȼ�С�ˣ�һ���Ǽ�����C��Ũ�ȣ�
�ʴ�Ϊ����СC��Ũ�ȣ�
��4��4minʱ����Ӧ��ƽ�⣬��ʱc��A��=0.4mol/L��c��B��=1.8mol/L��c��C��=1.2mol/L��
����ƽ�ⳣ��k=
 =
= =
= mol-1?L��
mol-1?L���ʴ�Ϊ��
 mol-1?L��
mol-1?L����5����4�������Ȼ�ѧ����ʽ���裺��Ӧ���ʱ�ֵΪX��
A��g��+2B��g��
 2C��g����H=-XkJ/mol
2C��g����H=-XkJ/mol1mol XkJ
��1mol/l-0.4mol/l��×1L=0.6mol 235.92kJ
����
 �����X=393.2��
�����X=393.2�����Է�Ӧ�ȡ�H=-393.2kJ/mol��
�ʴ�Ϊ��-393.2kJ/mol��
���������⿼����ѧ��Ӱ�컯ѧ��Ӧ���ʵ����غ��йط�Ӧ���ʵļ��㣬��һ�����ۺ��ԣ�

��ϰ��ϵ�д�
�����Ŀ
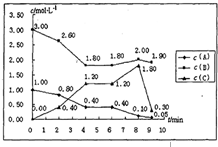 ��һ���ݻ��̶�Ϊ1L���ܱ������У�������ӦmA��g��+nB��g���TpC��g����H=������Ӧ�������ͼ��ʾ��
��һ���ݻ��̶�Ϊ1L���ܱ������У�������ӦmA��g��+nB��g���TpC��g����H=������Ӧ�������ͼ��ʾ�� CO2(g) + H2(g) ��H1=" +" 41.2kJ/mol
CO2(g) + H2(g) ��H1=" +" 41.2kJ/mol