��Ŀ����
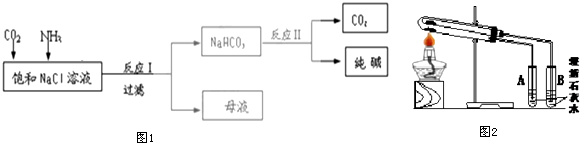
11�������λش�����������⣺��1����������������P4O6��P4O10�������ģ�ͽṹ��ͼ1��ʾ��������Ԫ�ص��ӻ����ͷֱ���sp3��sp3��
��2���������ֺ�����H3PO2��H3PO3��H3PO4��������Ԫ�ؾ���sp3�ӻ�������ԭ���γ��ĸ��Ҽ�����H3PO3�Ľṹʽ��
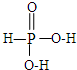 ��д��H3PO2����������������Һ��Ӧ�Ļ�ѧ����ʽNaOH+H3PO2=Na H2PO2+H2O�������ֺ�����H3PO2��H3PO3��H3PO4������ǿ��˳��ΪH3PO2��H3PO3��H3PO4����ԭ����H3PO2��H3PO3��H3PO4����Ԫ�صĻ��ϼ�����Ϊ+1��+3��+5�ۣ�������ԭ�ӵ�������������ߣ�����P-O-H��O�ĵ��Ӹ�����Pƫ�ƣ����H3PO2��H3PO3��H3PO4Խ����������ӣ�����Խ��Խǿ��
��д��H3PO2����������������Һ��Ӧ�Ļ�ѧ����ʽNaOH+H3PO2=Na H2PO2+H2O�������ֺ�����H3PO2��H3PO3��H3PO4������ǿ��˳��ΪH3PO2��H3PO3��H3PO4����ԭ����H3PO2��H3PO3��H3PO4����Ԫ�صĻ��ϼ�����Ϊ+1��+3��+5�ۣ�������ԭ�ӵ�������������ߣ�����P-O-H��O�ĵ��Ӹ�����Pƫ�ƣ����H3PO2��H3PO3��H3PO4Խ����������ӣ�����Խ��Խǿ����3����һ�ֻ�����������һ���ܵ��߶ȹ�ע����ĥͿ�ϣ��������������ı��汣���㣮����������廯������廯���������и��·�Ӧ�ϳɣ����廯����Ӻ����廯���ӵ����幹�ͷֱ���ƽ�������Ρ�������
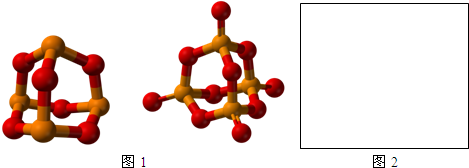
��4�����н����⣨Mo�������γɵ������A����ˮ��Һ����ȫ���룬����һ�ּ������Ӻ�һ�������ӣ�������Ϊ4��1�����������ӽṹ�������壬ʮ�ֶԳƣ���������������Զ����ԭ�ӵ�����Ϊ�����ӵ������ᣬ��������������Զ��Moԭ�ӵ�����Ϊ�����ӵ������ᣨn�����ʾij�����ƴ�����ת360��/n�ĽǶȣ����ܲ�����Ƿ���ת������
��д�������A�Ļ�ѧʽ[Mo6Cl8]Cl4��
����ͼ2�л���A��Һ�������ӵĽṹ��
�۰�һ�ֳ������ȵĶ�Ԫ������ľ���ȥ�����е���ԭ�ӣ�����ʣ�ಿ����A�������ӵĽṹ��ʮ�����ƣ����ֳ�����Ԫ��������NaCl��
���� ��1�����ݱ��֣�Pauling�����ӻ�������ۣ��۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ������Ҽ�����=��ԭ�Ӹ������ݴ��ж���ԭ�ӵ��ӻ���ʽ��
��2��������Ԫ�ؾ���sp3�ӻ�������ԭ���γ��ĸ��Ҽ��жϣ������������ӵĽṹʽ��H3PO2ΪһԪ���ᣬ�����ֺ�����H3PO2��H3PO3��H3PO4��������ԭ�ӵ�������������ߣ�����P-O-H��O�ĵ��Ӹ�����Pƫ�ƣ�Ӱ��������ǿ����
��3�������ӻ�������۷�����ԭ�Ӻ���ԭ�ӵļ۲���Ӷ����жϷ��ӹ��ͣ�
��4��������ЃȽ�ԭ�Ӳ��ܷ������룬���������ˮ��Һ���ܷ������룬�����⣨Mo�������γɵ������A����ˮ��Һ����ȫ���룬����һ�ּ������Ӻ�һ�������ӣ�������Ϊ4��1���������Ӻ��������֮��Ϊ1��4�������ӽṹ�������壬ʮ�ֶԳƣ���������������Զ����ԭ�ӵ�����Ϊ�����ӵ������ᣬ��������������Զ��Moԭ�ӵ�����Ϊ�����ӵ������ᣬ����8��Cl�������溬��Mo����һ�ֳ������ȵĶ�Ԫ������ľ���ȥ�����е���ԭ�ӣ�ʣ�ಿ����A�������ӵĽṹ��ʮ�����ƣ�Ϊ�Ȼ��ƽṹ���ݴ˷�����
��� �⣺��1�����ģ�ͽṹ�к��������ԭ�ӣ����������ԭ�ӣ�P4O6������ÿ����ԭ���γ�3���Ҽ���1�µ��Ӷԣ�������ԭ�ӵ��ӻ���ʽΪsp3��P=O˫������һ�Ҽ���P4O10������ÿ����ԭ���γ�4���Ҽ����µ��Ӷԣ�������ԭ�ӵ��ӻ���ʽΪsp3��
�ʴ�Ϊ��sp3�� sp3��
��2��H3PO3������Ԫ��������ԭ���γ��ĸ��Ҽ�����sp3�ӻ�����������-OH�����������γ�һ��������һ��˫��������H3PO3�Ľṹʽ��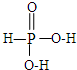 ���������Ƕ�Ԫ�ᣬ������ĵ���Ϊ��H3PO3?H++H2PO3-��H2PO3-?H++HPO32-��������������NaOH��Һ��Ӧ������Na2HPO3���÷�Ӧ����ʽΪ��NaOH+H3PO2=Na H2PO2+H2O��H3PO2��H3PO3��H3PO4����Ԫ�صĻ��ϼ�����Ϊ+1��+3��+5�ۣ�������ԭ�ӵ�������������ߣ�����P-O-H��O�ĵ��Ӹ�����Pƫ�ƣ����H3PO2��H3PO3��H3PO4Խ����������ӣ�����Խ��Խǿ��
���������Ƕ�Ԫ�ᣬ������ĵ���Ϊ��H3PO3?H++H2PO3-��H2PO3-?H++HPO32-��������������NaOH��Һ��Ӧ������Na2HPO3���÷�Ӧ����ʽΪ��NaOH+H3PO2=Na H2PO2+H2O��H3PO2��H3PO3��H3PO4����Ԫ�صĻ��ϼ�����Ϊ+1��+3��+5�ۣ�������ԭ�ӵ�������������ߣ�����P-O-H��O�ĵ��Ӹ�����Pƫ�ƣ����H3PO2��H3PO3��H3PO4Խ����������ӣ�����Խ��Խǿ��
�ʴ�Ϊ��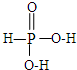 �� NaOH+H3PO2=Na H2PO2+H2O��H3PO2��H3PO3��H3PO4����Ԫ�صĻ��ϼ�����Ϊ+1��+3��+5�ۣ�������ԭ�ӵ�������������ߣ�����P-O-H��O�ĵ��Ӹ�����Pƫ�ƣ����H3PO2��H3PO3��H3PO4Խ����������ӣ�����Խ��Խǿ��
�� NaOH+H3PO2=Na H2PO2+H2O��H3PO2��H3PO3��H3PO4����Ԫ�صĻ��ϼ�����Ϊ+1��+3��+5�ۣ�������ԭ�ӵ�������������ߣ�����P-O-H��O�ĵ��Ӹ�����Pƫ�ƣ����H3PO2��H3PO3��H3PO4Խ����������ӣ�����Խ��Խǿ��
��3�����廯���������ԭ�ӵļ۲���Ӷ���Ϊ$\frac{3+3}{2}$=3��Bԭ�Ӱ�sp2��ʽ�ӻ���û�йµ��Ӷԣ����Է��ӿռ乹��Ϊƽ�������Σ��ṹʽΪ �����廯��������ԭ�ӵļ۲���Ӷ���Ϊ$\frac{5+3}{2}$=4��Pԭ�Ӱ�sp3��ʽ�ӻ�����һ�Թµ��Ӷԣ����Է��ӿռ乹��Ϊ�����Σ��ṹʽΪ
�����廯��������ԭ�ӵļ۲���Ӷ���Ϊ$\frac{5+3}{2}$=4��Pԭ�Ӱ�sp3��ʽ�ӻ�����һ�Թµ��Ӷԣ����Է��ӿռ乹��Ϊ�����Σ��ṹʽΪ ��
��
�ʴ�Ϊ��ƽ�������Σ�������
��4����������ЃȽ�ԭ�Ӳ��ܷ������룬���������ˮ��Һ���ܷ������룬�����⣨Mo�������γɵ������A����ˮ��Һ����ȫ���룬����һ�ּ������Ӻ�һ�������ӣ�������Ϊ4��1���������Ӻ��������֮��Ϊ1��4�������ӽṹ�������壬ʮ�ֶԳƣ���������������Զ����ԭ�ӵ�����Ϊ�����ӵ������ᣬ��������������Զ��Moԭ�ӵ�����Ϊ�����ӵ������ᣬ����8��Cl�������溬��Mo�����������A�Ļ�ѧʽΪ��[Mo6Cl8]Cl4��
�ʴ�Ϊ��[Mo6Cl8]Cl4��
��A��Һ��������Ϊ��[Mo6Cl8]4+������8��Cl�����������8�����㣬����6��Mo��������������ģ����������ӵĽṹΪ�� ��
��
�ʴ�Ϊ�� ��
��
���Ȼ��ƾ����ṹΪ�� ����X��Y��Z�����и�ķ���֪��X������2�������ӣ�Y������2�������ӣ�Z������2�������ӣ������ӵ���λ����6��ȥ�����е���ԭ�ӣ�ʣ�ಿ����A�������ӵĽṹ���ƣ�
����X��Y��Z�����и�ķ���֪��X������2�������ӣ�Y������2�������ӣ�Z������2�������ӣ������ӵ���λ����6��ȥ�����е���ԭ�ӣ�ʣ�ಿ����A�������ӵĽṹ���ƣ�
�ʴ�Ϊ��NaCl��
���� ���⿼�������ʽṹ���ۺ���֪ʶ�����ӵĿռ乹�͡��ӻ���֪ʶ���Ǹ߿����ȵ㣬Ӧ�ص����գ����������Ϣ������֪ʶ�ǽ��ؼ�����Ŀ�Ѷ��еȣ�

| A�� | �� | B�� | �� | C�� | �� | D�� | �� |
| A�� | 10 mL 0.1 mol/L��ˮ��10 mL 0.1 mol/L�����ϣ�c��Cl-��c��NH4+����c��OH-����c��H+�� | |
| B�� | 10 mL 0.1 mol/L NH4Cl��Һ��5mL 0.2 mol/L NaOH��Һ��ϣ�c��Na+��=c��Cl-����c��OH-����c��H+�� | |
| C�� | 10 mL 0.1 mol/L CH3COOH��Һ��5 mL 0.2 mol/L NaOH��Һ��ϣ�c��Na+��=c��CH3COO-����c��OH-����c��H+�� | |
| D�� | 10 mL 0.5 mol/L CH3COONa��Һ��6 mL 1 mol/L�����ϣ�c��Cl-����c��Na+����c��OH-����c��H+�� |
| ���� | ��� | �۵� | ȼ����/��kJ•mol-1�� |
| ���ʯ | ��ɫ�������� | �� | 395.4 |
| ʯī | �Һڣ��������� | �� | 393.5 |
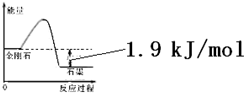
| A�� | �ɱ�����Ϣ�ɵ�����ͼ��ʾ��ͼ�� | |
| B�� | �ɱ�����Ϣ֪C��ʯī��s��=C�����ʯ��s����H=+1.9kJ/mol | |
| C�� | �ɱ�����Ϣ����֪��ͬ�����½��ʯ���۵����ʯī���۵� | |
| D�� | ��ʾʯīȼ���ȵ��Ȼ�ѧ����ʽΪC��ʯī��s��+$\frac{1}{2}$O2��g��=CO��g����H=-393.5kJ/mol |
| A�� | �������ĵ��뷽��ʽ��Al2��SO4��3�TAl3++SO42- | |
| B�� | �Ȼ��Ƶĵ���ʽ�� | |
| C�� | ����Ľṹ��ʽ��CH4 | |
| D�� | 14C��ԭ�ӽṹʾ��ͼ�� |