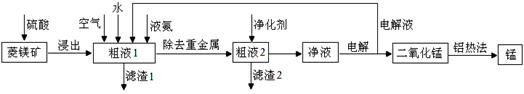
��Ŀ����
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ������
��ش��������⣺
��1����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
�ٵ�����X���ϵĵ缫��ӦʽΪ ��
��X�������۲쵽�������� ����
��Y�缫�ϵĵ缫��ӦʽΪ ��
����õ缫��Ӧ����ķ����� ��
��2����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����
��X�缫�IJ����� ���缫��Ӧʽ�� ��
��Y�缫�IJ����� ���缫��Ӧʽ�� ��
��˵�������ʷ����ĵ缫��Ӧ����д����
�Ţ�2H+ + 2e - = H2����2�֣� �ų����壬��Һ��졣��2�֣�
��2Cl - - 2e - = Cl2 ����2�֣�
��ʪ��ĵ⻯�ص�����ֽ����Y�缫��������ֽ����ɫ����2�֣�
�Ƣٴ�ͭ����ͭ����1�֣� Cu2+ + 2e - =" Cu" ��2�֣�
�ڴ�ͭ��1�֣� Cu - 2e - = Cu2+ ��2�֣�
���������������1��X��Ϊ�����������ӷŵ磬������������X��������������ˮ������������������ӣ�������̪����Һ��죻Y��Ϊ�����������ӷŵ磬���������������ļ��鷽��Ϊ����ʪ��ĵ⻯�ص�����ֽ����Y�缫��������ֽ����ɫ����2��������ͭ������Ϊ��ͭ����X����������ԭ��Ӧ���缫��ӦʽΪCu2+ + 2e - = Cu������Ϊ��ͭ����Y��������������Ӧ���缫��ӦʽΪCu - 2e - = Cu2+��
���㣺����
����������������߿�����֪ʶ�㣬ע�ؿ���ѧ���������⡢��������������

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д��±���ʵ������ܴﵽʵ��Ŀ�ĵ���
| | ʵ����� | ʵ��Ŀ�� |
| A | �ӵı�����Һ�еμ�ϡ��ˮ | ��֤���屽��Ϊ��ɫ���� |
| B | ���������Һ�м����Ƶ�Cu(OH)2����Һ������ | ȷ���������к���ȩ�� |
| C | ��ƾ�������Ļ��Һ�м�������� | ȷ���ƾ��л��д��� |
| D | ��������������������Һ����һ��ʱ�䣬������ȴ��Ļ��Һ�еμ���������Һ | ����ˮ������е������� |


 ��
��

 ��
�� ��Һ������
��Һ������ ���ӷ���ת�ƣ��Իش��������⣺
���ӷ���ת�ƣ��Իش��������⣺ �������� ���õ�
�������� ���õ� ���������״������ ��
���������״������ �� �����С����������䡱��
�����С����������䡱��