��Ŀ����
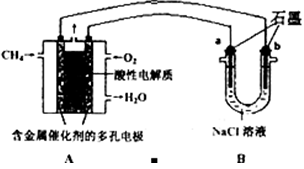
����ͼװ���У�b�缫�ý��� M�Ƴɣ�a��c��dΪʯī�缫����ͨ��Դ������M������b����ͬʱa��d�缫�ϲ������ݡ��Իش�
��1��aΪ ����c���ĵ缫��ӦʽΪ ��
��2��������һ��ʱ�������c���ϵ��Թ���Ҳ���ռ��������壬��ʱc���ϵĵ缫��ӦʽΪ ��
��3����d�����ռ���44.8mL���壨��״����ʱֹͣ��⣬a���Ϸų�����������ʵ���Ϊ ����b�缫�ϳ�������M������Ϊ0.432g����˽�����Ħ������Ϊ ��
��1������1�֣��� 2I��һ2e����I2��2�֣�
��2��40H����4e����2H2O+O2�� ��2�֣�
��3��0��001 mol��1�֣���108g��mol ��2�֣�
��������������ɵ��ԭ���ɵã�����M������b����˵��b����������a��������c��������d����������1����a����������Һ�е������ӷŵ磬�������ӵķŵ�˳��֪��2I--2e-=I2����2����B�ձ��У� c����������Һ�е������ӷŵ磬��2I--2e-=I2��I2����������ʹ���۱�����I-�ŵ���Ϻ�����OH-�ŵ磺4OH--4e=2H2O+O2����c���ϵ��Թ����ռ���������Ϊ��������3��d�缫���ռ���44.8ml���壨��״������������a�����ռ���������������������ת�Ƶ��������֪�����������������֮����1��2��d�缫���ռ���44.8ml������������a�缫���ռ���22.4mL���������ʵ���Ϊ0.01mol��d�缫�����������������ʵ���= ��ת�Ƶ��ӵ����ʵ�����0.04mol����������M��+1�ۣ����Ե�ת��0.04mol����ʱ����0.04mol�������ʣ�M=
��ת�Ƶ��ӵ����ʵ�����0.04mol����������M��+1�ۣ����Ե�ת��0.04mol����ʱ����0.04mol�������ʣ�M=
���㣺ԭ��غ͵��صĹ���ԭ����

ij��ѧ��ȤС��Ϊ��̽�����缫��ԭ����е�����,��Ʋ�����������һϵ��ʵ��,ʵ������¼����:
| ��� | �缫���� | �������Һ | ������ָ�� ƫת���� |
| 1 | Mg��Al | ϡ���� | ƫ��Al |
| 2 | Al��Cu | ϡ���� | ƫ��Cu |
| 3 | Al��ʯī | ϡ���� | ƫ��ʯī |
| 4 | Mg��Al | NaOH��Һ | ƫ��Mg |
| 5 | Al��Zn | Ũ���� | ƫ��Al |
�����ϱ��е�ʵ�����������������:
��1��ʵ��1��2��Al�����ĵ缫�Ƿ���ͬ?����������������������������
��2��ָ��ʵ��3������ʯī�ĵ缫���ƣ���д��ʵ���еĵ缫��Ӧ�͵���ܷ�Ӧ��
��Ϊ(����)�� �� �� �� ���� ʯīΪ(����)�� �� �� �� ����
����ܷ�Ӧ: ��
��3��ʵ��4�е������������Ǹ���?������,Ϊʲô?����������������������������
��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��109g5.51����NaOH��Һ��������CuSO4��Һ��200g10.00����K2SO4��Һ���缫��Ϊʯī�缫��
��ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47��������c�缫�������ӡ��ݴ˻ش����⣺
��1���缫b�Ϸ����ĵ缫��ӦΪ___________________________________��
��2���缫b�����ɵ������ڱ�״���µ����Ϊ__________________����ʱ���ձ���NaOH��Һ�����ʵ���Ũ��Ϊ������Һ���ܶ�Ϊ1g/cm3��_______________��
��3���缫c�������仯��___________g����ʹ�������еĵ��Һ�ָ�����ʼ״̬��Ӧ������Һ�м���������___________������ĸ��ţ���
| A��Cu(OH)2 | B��Cu2O | C��CuCO3 | D��Cu2(OH)2CO3 |
��֪̼̼�������Ƽ���������ת,ij���Ľṹ��ʽ����ͼ��ʾ,����˵������ȷ����
| A������������ԭ�Ӿ��ɹ��� |
| B��������������10��̼ԭ�Ӵ���ͬһƽ���� |
| C��������������11��̼ԭ�Ӵ���ͬһƽ���� |
| D�������뱽��Ϊͬϵ�� |






 2CO2(g)+ N2(g)��H <0
2CO2(g)+ N2(g)��H <0
 N2O4(g) ��H����56.9 kJ/mol ��
N2O4(g) ��H����56.9 kJ/mol ��