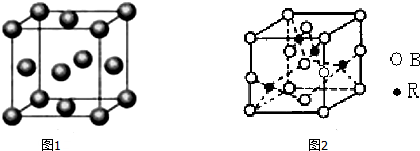
��Ŀ����
20�����Т�CaBr2 �ڶ������� ��Ar ��ͭ �ݣ�NH4��2SO4 �ɱ� �߳����µĴ��������� �Ȱ������ʣ�������Ҫ��ش�����ţ���1���������Ӿ�����Ǣ٢ݣ� ���ڷ��Ӿ�����Ǣۢޢߢ࣮
��2��������ֻ����һ�������������Ǣ٢ڢܣ�
��3�������ۻ�ʱ��Ҫ�ƻ����ۼ����Ǣڣ�
��4�������мȺ����Ӽ����ֺ����ۼ����Ǣݣ�
��5�����岻���磬��ѹ���ۻ�ʱ�ܵ�����Ǣ٢ݣ�
���� ��1���������Ӽ��ľ��������Ӿ��壬���Ӿ����п��ܺ��й��ۼ�������ͨ�����Ӽ���������ϵ����ڷ��Ӿ��壻
��2��������ֻ����һ��������������˵��������Ϊԭ�Ӿ����ԭ�ӷ��ӵķ��Ӿ����ֻ�����Ӽ������Ӿ����������壻
��3��ԭ�Ӿ����ۻ�ʱ�ƻ����ۼ���
��4���������Ӽ��ľ��������Ӿ��壬���Ӿ����п��ܺ��й��ۼ���
��5�����Ӿ�����岻���磬��ѹ���ۻ�ʱ�ܵ��磮
��� �⣺��CaBr2 �������Ӿ��壬ֻ�������Ӽ���
�ڶ��������д��ڹ��ۼ�������ԭ�Ӿ��壻
��Ar������û�й��ۼ���ֻ�з��Ӽ������������ڷ��Ӿ��壻
��ͭ �ǽ������壬������ֻ����һ�������������ǽ�������
�ݣ�NH4��2SO4�����мȺ����Ӽ����ֺ����ۼ���
�ɱ����Ӵ��ڹ��ۼ��ͷ��Ӽ������������ڷ��Ӿ��壻
�߳����µĴ�������ڹ��ۼ��ͷ��Ӽ������������ڷ��Ӿ��壻
����״��ڹ��ۼ��ͷ��Ӽ������������ڷ��Ӿ��壻
��1���������Ӽ��ľ��������Ӿ��壬���Ӿ����п��ܺ��й��ۼ����������Ӿ�����Ǣ٢ݣ�����ͨ�����Ӽ���������ϵ����ڷ��Ӿ��壬��ۢޢߢ����ڷ��Ӿ��壻
�ʴ�Ϊ���٢ݣ��ۢޢߢࣻ
��2��������ֻ����һ��������������˵��������Ϊԭ�Ӿ����ԭ�ӷ��ӵķ��Ӿ����ֻ�����Ӽ������Ӿ����������壬��Ϊ�٢ڢܣ��ʴ�Ϊ���٢ڢܣ�
��3��ԭ�Ӿ����ۻ�ʱ�ƻ����ۼ�������ԭ�Ӿ����Ϊ���ڣ��ʴ�Ϊ���ڣ�
��4���������Ӽ��ľ��������Ӿ��壬���Ӿ����п��ܺ��й��ۼ����Ⱥ����Ӽ����ֺ����ۼ����Ǣݣ��ʴ�Ϊ���ݣ�
��5�����Ӿ�����岻���磬��ѹ���ۻ�ʱ�ܵ��磬���Թ��岻���磬��ѹ���ۻ�ʱ�ܵ�����Ǣ٢ݣ��ʴ�Ϊ���٢ݣ�
���� ���⿼���˾����д��ڵ������������ݾ���Ĺ�����������������������������Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | SiO2 CaO NaCl CBr4 CF4 | |
| B�� | SiO2 NaCl CaO CF4 CBr4 | |
| C�� | CaO NaCl SiO2 CBr4 CF4 | |
| D�� | CF4CBr4NaCl CaO SiO2 |
| A�� | ɽ�廬�� | B�� | ������ | C�� | �����ˮ | D�� | �ⵥ������ |
| A�� | ���� | B�� | ���� | C�� | �ᾧ | D�� | ��ȡ |
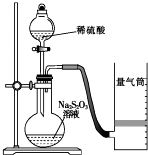 ��ѧ��Ӧ������������ѧ��Ӧ���п����̶ȵ���������������ijͬѧ�ⶨ��ѧ��Ӧ���ʲ�̽����Ӱ�����ص�ʵ�飮
��ѧ��Ӧ������������ѧ��Ӧ���п����̶ȵ���������������ijͬѧ�ⶨ��ѧ��Ӧ���ʲ�̽����Ӱ�����ص�ʵ�飮�ⶨ��ѧ��Ӧ����
��ͬѧ������ͼװ�òⶨ��ѧ��Ӧ���ʣ�
����֪��S2O32-+2H+=H2O+S��+SO2����
��1��Ϊ��֤ʵ��ȷ�ԡ��ɿ��ԣ����ø�װ�ý���ʵ��ǰӦ�Ƚ��еIJ����Ǽ��װ�õ������ԣ�����ͼ��ʾ��ʵ����Ʒ�������⣬����Ҫ��һ��ʵ�������������
��2������2minʱ�ռ���224mL��������ɱ�״�������壬�ɼ������2min��H+�ķ�Ӧ���ʣ����òⶨֵ��ʵ��ֵƫС����ԭ����SO2�Ჿ������ˮ�����������SO2���ƫС��
��3���Լ����ⶨ�÷�Ӧ�Ļ�ѧ��Ӧ���ʵ�����������дһ�֣����ⶨһ��ʱ�����������ʵ�������ⶨһ��ʱ������ҺH+Ũ�ȵı仯
��Ϊ̽�ֻ�ѧ��Ӧ���ʵ�Ӱ�����أ���Ƶ�ʵ�鷽�������
| ʵ����� | ���V/mL | ʱ��/s | |||
| Na2S2O3��Һ | ������Һ | ��ˮ | ˮ | ||
| �� | 10.0 | 2.0 | 4.0 | 0.0 | t1 |
| �� | 8.0 | 2.0 | 4.0 | 2.0 | t2 |
| �� | 6.0 | 2.0 | 4.0 | Vx | t3 |
��4����ʵ����е�Ŀ����̽����Ӧ��Ũ�ȣ�Na2S2O3���Ի�ѧ��Ӧ���ʵ�Ӱ�죬������Һ����������Ϊ��ɫ��������I2�Ĵ��ڣ�����Vx=4.0 mL���Ƚ�t1��t2��t3��С�����Ʋ��ʵ����ۣ������������䣬��Ӧ��Ũ��Խ��ѧ��Ӧ����Խ��