��Ŀ����
1����ͬ�¶��£�������ѹ�ܱ������з������淴ӦX2��g��+3Y2��g��?2XY3��g����H=-92.6kJ•mol-1ʵ���÷�Ӧ����ʼ���ﵽƽ��ʱ���й����������ʾ��| ������� | ��ʼʱ���������ʵ���/mol | ��ƽ��ʱ��ϵ�����仯 | ||
| X2 | Y2 | XY3 | ||
| �� | 0.1 | 0.3 | 0 | 4.63kJ |
| �� | 0.8 | 2.4 | 0.4 | Q��Q��0��kJ |
| A�� | ��Ӧ��ƽ�ⳣ�����٣��� | |
| B�� | ��ƽ��ʱ������������XY3�����ʵ���Ũ�Ⱦ�Ϊ2mol•L-1 | |
| C�� | �ﵽƽ��ʱ�������١����и����ʵİٷֺ�����ͬ | |
| D�� | �����������Ϊ0.20L�����ƽ��ʱ�ų�����������4.63kJ |
���� A��ƽ�ⳣ��ֻ���¶��йأ��¶���ȣ�ƽ�ⳣ����ȣ�
B���������а���ѧ������ת������߿ɵ�X20.2mol��Y20.6mol������X2����Ϊ1mol��Y2����Ϊ3mol��n��X2����n��Y2��=1��3���¶Ⱥ�ѹ�£������١����дﵽƽ��Ϊ��Чƽ�⣬ƽ��ʱ��ͬ���ʵ�Ũ����ͬ���������δ֪������ȷ������Ũ�ȣ�
C���¶Ⱥ�ѹ�£������١����дﵽƽ��Ϊ��Чƽ�⣬ƽ��ʱ��ͬ���ʵĺ�����ͬ��
D�������ٴ�ƽ��ʱ�ų�������4.63kJ�����ɵ�XY3�����ʵ���Ϊ$\frac{4.63kJ}{92.6kJ}$��2mol=0.1mol����֪����ʼ��������ж�ƽ��ʱXY3�����ʵ�����0.1mol��ϵ��
��� �⣺A��ƽ�ⳣ��ֻ���¶��йأ��¶Ȳ��䣬��ͬһ��Ӧƽ�ⳣ�����䣬��A����
B���������а���ѧ������ת������߿ɵ�X20.2mol��Y20.6mol������X2����Ϊ1mol��Y2����Ϊ3mol��n��X2����n��Y2��=1��3�����������ж�Ӧ���ʵ����ʵ�������ͬ���¶Ⱥ�ѹ�£������١����дﵽƽ��Ϊ��Чƽ�⣬ƽ��ʱ����������XY3�����ʵ���Ũ����ͬ����֪����ʼ�����������ƽ��ʱ��������ʲ��ܵõ������Ũ�ȣ���B����
C���¶Ⱥ�ѹ�£������١����дﵽƽ��Ϊ��Чƽ�⣬ƽ��ʱ��ͬ���ʵĺ�����ͬ����C��ȷ��
D�����º�ѹ�£������������е����ʵ�ת����һ������ƽ��ʱ�ų�������4.63kJ�����ɵ�XY3�����ʵ���Ϊ$\frac{4.63kJ}{92.6kJ}$��2mol=0.1mol����֪����ʼ��������ж�ƽ��ʱXY3�����ʵ�����0.1mol��ϵ�����жϴ�ƽ��ʱ�ų���������4.63kJ��ϵ����D����
��ѡ��C��
���� ���⿼�黯ѧƽ���ƶ����⡢��Чƽ������⣬��Ŀ�Ѷ��еȣ��ؼ��ǵ�Чƽ����ɵ��������գ�

 ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
�㽭֮��ѧҵˮƽ����ϵ�д�| A�� | ͬŨ�ȡ�ͬ�����ǿ����ǿ����Һ��Ϻ���Һ��pH=7 | |
| B�� | ��10mL pH=a��������100mL pH=b��Ba��OH��2��Һ��Ϻ�ǡ���кͣ���a+b=13 | |
| C�� | ��pH=10��Ba��OH��2��Һ��pH=13��NaOH��Һ�������ϣ���Ϻ���Һ��pH=10.7����֪lg2=0.3�� | |
| D�� | pH=2��������pH=12�İ�ˮ�������Ϻ�������ҺpH=7 |
| A�� | 2N2H4��g��+N2O4��g���T3N2��g��+4H2O��g����H=-542.7 kJ/mol | |
| B�� | 2N2H4��g��+N2O4��g���T3N2��g��+4H2O��g����H=-1 059.3 kJ/mol | |
| C�� | 2N2H4��g��+N2O4��g���T3N2��g��+4H2O��g����H=-1 076.7 kJ/mol | |
| D�� | N2H4��g��+$\frac{1}{2}$N2O4��g���T$\frac{3}{2}$N2��g��+2H2O��g����H=-1 076.7 kJ/mol |
��1����CH4����ԭ��������������������������Ⱦ����֪��
��CH4 ��g��+4NO2 ��g��=4NO��g��+CO2 ��g��+2H2 O��g����H=-574kJ•mol-1
��CH4 ��g��+4NO��g��=2N2 ��g��+CO2 ��g��+2H2 O��g����H=-1160kJ•mol-1
��H2O��g��=H2O��l����H=-44.0kJ•mol-1
д��CH4��g����NO2��g����Ӧ����N2��g����CO2��g����H2O��l�����Ȼ�ѧ����ʽCH4��g��+2NO2��g��=N2��g��+CO2��g��+2H2O��l����H=-955kJ•mol-1
��2���û���̿��ԭ��������������йط�ӦΪ��C��s��+2NO��g��?N2 ��g��+CO2 ��g��ij�о�С��������ܱ���������һ���Ļ���̿��NO�����������·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ������
 | NO | N2 | CO2 |
| 0 | 0.100 | 0 | 0 |
| 10 | 0.058 | 0.021 | 0.021 |
| 20 | 0.040 | 0.030 | 0.030 |
| 30 | 0.040 | 0.030 | 0.030 |
| 40 | 0.032 | 0.034 | 0.017 |
| 50 | 0.032 | 0.034 | 0.017 |
A��������CO2��Ũ�ȱ��ֲ��� B��v����N2��=2v����NO��
C��������ѹǿ���ֲ��� D�����������ܶȱ��ֲ���
E����������ƽ����Է����������ֲ���
���ڸ��¶���ʱ���÷�Ӧ��ƽ�ⳣ��Ϊ0.56��������λС������
����30min���ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı�������Ǽ�С������̼Ũ�ȣ�
��3���ϳ���ʧȥ���Եĸ�п��ý�������Ʊ�п�̵�أ��ڼ��������£��õ�ص��ܷ�ӦΪ��
Zn��s��+2MnO2 ��s��+H2O��l��=Zn��OH��2 ��s��+Mn2O3��s�����õ�������ĵ缫��Ӧʽ��2MnO2+2e-+H2O=2OH-+Mn2O3��PbO2 ����ͨ��ʯīΪ�缫��Pb��NO3��2��Cu��NO3��2�Ļ����ҺΪ���Һ�����ȡ������������Ӧ�ĵ缫��ӦʽΪPb2++2H2O-2e-=PbO2+4H+�������Ϲ۲쵽�������ǣ�ʯī�Ϻ�ɫ�������������Һ�в�����Cu��NO3��2����������Ҫȱ����Pb��������������Pb2+���ܵõ���ЧӦ�ã�
ѹǿ/MPa �������/% �¶�/�� | 1.0 | 2.0 | 3.0 |
| 810 | 46.0 | a | b |
| 915 | c | 25.0 | d |
| 1000 | e | f | 15.0 |
��1�������C�IJ��ʣ�Ӧ��ȡ�Ĵ�ʩΪ�����¶ȡ�����ѹǿ��
��2���÷�Ӧ��H�� 0���������������
��3���Ƚ�K��810�棩�� K��915�棩 �����������������=������˵�����������¶�ƽ���������ƶ������ƽ�ⳣ������
��4��915��2.0MPʱA��ת����Ϊ60%��
��5���Ƚ�b��f�����������������=������˵����������ƽ�������ƶ�����f��25.0��a������ѹǿƽ�������ƶ�����a��b������b��f��
��6����1000�棬3.0MPaʱ���������м���0.16mol A��0.20mol B��ƽ��ʱ�������Ϊ10L�����ʱƽ�ⳣ��K=0.133��������λ��Ч���֣���
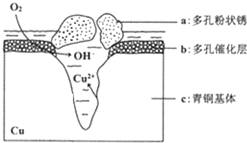 �ҹ��Ŵ���ͭ�����վ�տ���кܸߵ�������ֵ����ʷ��ֵ������������ͭ������ܵ�������ʴ���ʶ���������ͷ���������Ҫ���壮 ��ͼΪ��ͭ���ڳ�ʪ�����з����绯ѧ��ʴ��ԭ��ʾ��ͼ������˵������ȷ���ǣ�������
�ҹ��Ŵ���ͭ�����վ�տ���кܸߵ�������ֵ����ʷ��ֵ������������ͭ������ܵ�������ʴ���ʶ���������ͷ���������Ҫ���壮 ��ͼΪ��ͭ���ڳ�ʪ�����з����绯ѧ��ʴ��ԭ��ʾ��ͼ������˵������ȷ���ǣ�������| A�� | ��ʴ�����У�������a | |
| B�� | ������Ӧ�� O2+4e-+2H2O�T4OH- | |
| C�� | ������4.29 g Cu2��OH��3Cl���������Ϻ������Ϊ0.224L����״���� | |
| D�� | �����е�Cl-��ɢ���ڣ�����������Ӧ���������Ӧ�����������ɶ��״��Cu2��OH��3Cl�������ӷ���ʽΪ2Cu2++3OH-+Cl-�TCu2��OH��3Cl�� |
| A�� | CH4+2 O2 $\stackrel{��ȼ}{��}$ CO2+2H2O | |
| B�� | CH2=CH2+H2 $\stackrel{����}{��}$ CH3-CH3 | |
| C�� |  +Br2 $\stackrel{FeBr_{3}}{��}$ +Br2 $\stackrel{FeBr_{3}}{��}$ +HBr +HBr | |
| D�� | 2CH3CH2OH+O2 $��_{��}^{Cu��Ag}$ 2CH3CHO+2 H2O |