��Ŀ����
16�� ����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У�
����ͼ��ʾװ�ý���ʵ�飬��A��μ���B�У���1����BΪNa2CO3��ĩ��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ�ɳ������ǣ����Թ�C�л�ѧ��Ӧ�Ļ�ѧ����ʽ��C6H5O-+CO2+H2O�TC6H5OH+HCO3-��Ȼ�����ձ��м����ˮ���ɹ۲쵽�Թ�C�е�������Һ���DZ���壮
��2����B����ʯ�ң��۲쵽C��Һ�����γɳ�����Ȼ������ܽ⣮��������ȫ�ܽ⣬ǡ�ñ����ʱ���ر�EȻ����С�Թ��м���������ȩ��Һ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������A��Ũ��ˮ�������ƣ���C��AgNO3���ѧʽ��������ȩ�Ļ�Ϻ���Һ�з�Ӧ�Ļ�ѧ����ʽ��CH3CHO+2[Ag��NH3��2]OH$\stackrel{��}{��}$CH3COONH4+2Ag��+3NH3+H2O��ʵ�����ʱ��ϡ������ϴ������
���� ��1��̼�����Աȱ���ǿ��������̼���뱽������Һ��Ӧ���ɱ��ӣ�
��2���۲쵽�Թܱڳ��ֹ�����������˵��C������������Һ����AӦΪŨ��ˮ������ʯ�����������ɰ�������������������Һ��Ӧ����������Һ��������Һ����ȩ�ڼ��������·�Ӧ����������
��� �⣺��1��CΪC6H5ONa��Һ��ʵ���й۲쵽С�Թ�����Һ�ɳ������ǣ�˵���б������ɣ�BΪCaCO3��ĩ������A��һ��ǿ�ᣬ��̼��Ʒ�Ӧ�ų�������̼��̼������Աȱ���ǿ����������̼ͨ�뵽�������л����ɱ��ӳ�������C6H5O-+H2O+CO2=C6H5OH+HCO3-�����¶ȵ���16.6��ʱΪ��ɫ���壬���ձ��м����ˮ�������ܽ�����¶����������ܽ����Ϊ��ɫҺ�壬
�ʴ�Ϊ��C6H5O-+H2O+CO2=C6H5OH+HCO3-����Һ���DZ���壻
��2����С�Թ��м���3����ȩ�������ձ��м�����ˮ������Ƭ�̣��۲쵽�Թܱڳ��ֹ�������������Ϊȩ�ܺ�������Һ֮�䷢��������Ӧ������������Һ�мӰ�ˮ��ͨ�������������պ���ʧʱ�����Ի��������Һ������A��Ũ��ˮ��C������������ȩ��������Һ���������ӦΪ��CH3CHO+2[Ag��NH3��2]OH$\stackrel{��}{��}$CH3COONH4+2Ag��+3NH3+H2O�����θ���ܾ��з��������ã����Dz����ý�������ϡ���ᣬ����ϡ������ϴ������
�ʴ�Ϊ��Ũ��ˮ��AgNO3��CH3CHO+2Ag��NH3��2OH$\stackrel{ˮԡ����}{��}$CH3COONH4+H2O+2Ag��+3NH3��ϡ���
���� ���⿼���Ϊ�ۺϣ��漰���ʵ�����̽���Լ��Ʊ���֪ʶ��Ϊ��Ƶ���㣬������ѧ���ķ���������ʵ�������Ŀ��飬ע��������ʵ������Լ�ʵ���ԭ�����������Ѷ��еȣ�

 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �ļ�������麟������������Ƭ���ù������ʹ��̼�����ҵ����ʯīΪ�缫����ļ��Ȼ����Һ�Ʊ��ļ�������� ��Һ��ԭ����ͼ��ʾ������˵������ȷ���ǣ�������
�ļ�������麟������������Ƭ���ù������ʹ��̼�����ҵ����ʯīΪ�缫����ļ��Ȼ����Һ�Ʊ��ļ�������� ��Һ��ԭ����ͼ��ʾ������˵������ȷ���ǣ�������| A�� | b��Ϊ������������ԭ��Ӧ | |
| B�� | a��������������ʹʪ��ĵ��۵⻯����ֽ���� | |
| C�� | ���·���ӵ�����ΪM��a��b��N | |
| D�� | ��ⷴӦ���ܻ�ѧ����ʽΪ2��CH3��4NCl+2H2O$\frac{\underline{\;���\;}}{\;}$2��CH3��4NOH+H2��+Cl2�� |
| A�� | H+��Na+��Cl- | B�� | H+��Ag+��Cl- | C�� | Na+��H+��NO3- | D�� | Fe2+��H+��NO3- |
��1����ȡ���������Լ�����5֧�Թ��У�������������ˮ�����Թܣ��۲쵽����������Һ����ɫ������������ʵĻ�ѧʽ��CuSO4
��2���ֱ���ʣ�µ�4����Һ���Թ��м��루1�����Ѽ������Һ���۲쵽���������Ӧ�Ļ�ѧ��Ӧ����ʽ�ǣ�
| ���� | ��ѧ��Ӧ����ʽ | |
| �� | �Թ����а�ɫ�������� | Ba��NO3��2+CuSO4�TBaSO4��+Cu��NO3��2 |
| �� | �Թ�������ɫ�������� | CuSO4+2NaOH�TCu��OH2����+2NaNO3 |
| A�� | �ᳫ���ǹ���ʱ����ʹ�����ϴ�����Ϊ�˼��ٰ�ɫ��Ⱦ | |
| B�� | ��ɫ��ѧ�ĺ��������û�ѧԭ����Դͷ�ϼ��ٺ�������ҵ�����Ի�������Ⱦ | |
| C�� | Ϊ��ֹ����е��ؽ�������Ⱦ������ˮ�壬Ӧ���м��������� | |
| D�� | ��������͵����������γ��������Ҫ���� |
| A�� | ԭ��������Z��Y��X��W | |
| B�� | X��Y��Z��W�γɵĵ��������6�� | |
| C�� | XԪ������������Ӧˮ����Ļ�ѧʽΪ��HXO3 | |
| D�� | ����Ԫ�ص���̬�⻯���У�W����̬�⻯�����ȶ� |
| A�� | ����������ˮ��2K+2H2O�T2K++OH-+H2�� | |
| B�� | С�մ���Һ�м�������NaOH��Һ��H++OH-�TH2O | |
| C�� | FeCl3��Һ��ͭ��Ӧ��Fe3++Cu�TFe2++Cu2+ | |
| D�� | ������Һ�м�������������Һ�����ԣ�Ba2++OH-+H++SO42-�TBaSO4��+H2O |
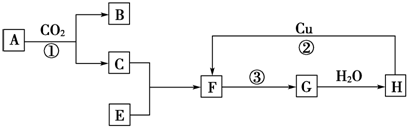
 ��������ֲ��ӷ����е�һ�ֳɷ֣����ں����ӵ�����˵����
��������ֲ��ӷ����е�һ�ֳɷ֣����ں����ӵ�����˵����