��Ŀ����
��״���£����мס��ҡ�������ʵ�飬��ȡ30.0mL��ͬŨ�ȵ�������Һ��Ȼ��ֱ������ؼ��벻ͬ������ͬһ��þ���Ͻ��ĩ���������й����ݣ������跴Ӧǰ����Һ������������仯����ش�
��1�������������ݣ�������������ʵ���Ũ��Ϊ mol/L��
��2�������������ݣ�����þ�������ʵ���֮��Ϊ ��
��3������ʵ����������м���һ����1mol/L��NaOH��Һ��ǡ��ʹ��Ԫ��ȫ����AlO2-��ʽ���ڣ���ʹMg2+�պó�����ȫ������NaOH���Ϊ ����
��4��ȡһ������Al2O3Ͷ�뵽NaOH��Һ�У�ȫ���ܽ⣬��ͨ��CO2���壬��ͨ��CO2����2.24Lʱ������11.7�˵ij��������ͨ��CO2����1.12Lʱ�����ɵij����Ƕ��ٿˣ�
| �� | �� | �� | |
| �Ͻ�����/mg | 255 | 385 | 459 |
| �������/mL | 280 | 336 | 336 |
��2�������������ݣ�����þ�������ʵ���֮��Ϊ
��3������ʵ����������м���һ����1mol/L��NaOH��Һ��ǡ��ʹ��Ԫ��ȫ����AlO2-��ʽ���ڣ���ʹMg2+�պó�����ȫ������NaOH���Ϊ
��4��ȡһ������Al2O3Ͷ�뵽NaOH��Һ�У�ȫ���ܽ⣬��ͨ��CO2���壬��ͨ��CO2����2.24Lʱ������11.7�˵ij��������ͨ��CO2����1.12Lʱ�����ɵij����Ƕ��ٿˣ�
���㣺�йػ���ﷴӦ�ļ���
ר�⣺������
����������Ũ�ȡ����һ�������кϽ�����С�����кϽ��������Ҽ��������������С���������������˵���������������������ȫ��Ӧ�����кϽ�����С�ڱ��кϽ����������ҡ����������������ȣ�˵���ҡ�����������ȫ��Ӧ������336mL������Ҫ����������Ϊ255mg��
=306mg��385mg�������н���ʣ�࣬����㣬
��1���ҡ�����������ȫ�����Ը��ݷ�Ӧ�����������������������ʵ���Ũ�ȣ�����n=
�������������ʵ�����������Ԫ���غ��֪n��HCl��=2n��H2�����ݴ˼��㣻
��2������������ʣ�࣬������ȫ��Ӧ����ʱ��������280mL���ʿ��Ը��ݼ������ݼ�����������ʵ���֮�ȣ���þ���������ʵ����ֱ�Ϊxmol��ymol�����ݶ�������֮�������ת���غ��з��̼���x��y��ֵ���ݴ˽��
��3������ʵ����������м���һ������NaOH��Һ��ǡ��ʹ��Ԫ��ȫ����AlO2-��ʽ���ڣ���ʹMg2+�պó�����ȫ����ʱ��Һ������ΪNaCl��NaAlO2�������������غ���n��NaOH��=n��NaCl��+n��NaAlO2�������Cl�����ӡ�Alԭ���غ��֪n��NaCl����n��NaAlO2�����ٸ���V=
������Ҫ����������Һ�������ϣ�1����2���е����ݼ��㣻
��4����ʼ���ܷ�����Ӧ��2NaOH+CO2=Na2CO3+H2O��Ȼ����������ķ�ӦΪ��2AlO2-+CO2+3H2O=2Al��OH��3��+CO32-��ͨ��CO2����2.24Lʱ������11.7�˵ij���������Ϊ���������������ʵ���=
=0.15��������̼���ʵ���Ϊ0.1mol�������ɳ���ʱ���Ķ�����̼Ϊ0.075mol������0.025mol������̼��NaOH��Ӧ����֪ʣ����������Ϊ0.05mol���ݴ˽�Ϸ���ʽ���㣮
| 336mL |
| 280mL |
��1���ҡ�����������ȫ�����Ը��ݷ�Ӧ�����������������������ʵ���Ũ�ȣ�����n=
| V |
| Vm |
��2������������ʣ�࣬������ȫ��Ӧ����ʱ��������280mL���ʿ��Ը��ݼ������ݼ�����������ʵ���֮�ȣ���þ���������ʵ����ֱ�Ϊxmol��ymol�����ݶ�������֮�������ת���غ��з��̼���x��y��ֵ���ݴ˽��
��3������ʵ����������м���һ������NaOH��Һ��ǡ��ʹ��Ԫ��ȫ����AlO2-��ʽ���ڣ���ʹMg2+�պó�����ȫ����ʱ��Һ������ΪNaCl��NaAlO2�������������غ���n��NaOH��=n��NaCl��+n��NaAlO2�������Cl�����ӡ�Alԭ���غ��֪n��NaCl����n��NaAlO2�����ٸ���V=
| n |
| c |
��4����ʼ���ܷ�����Ӧ��2NaOH+CO2=Na2CO3+H2O��Ȼ����������ķ�ӦΪ��2AlO2-+CO2+3H2O=2Al��OH��3��+CO32-��ͨ��CO2����2.24Lʱ������11.7�˵ij���������Ϊ���������������ʵ���=
| 11.7g |
| 78g/mol |
���
�⣺����Ũ�����һ�������кϽ�����С�����кϽ��������Ҽ��������������С���������������˵���������������������ȫ��Ӧ�����кϽ�����С�ڱ��кϽ����������ҡ����������������ȣ�˵���ҡ�����������ȫ��Ӧ������336mL������Ҫ����������Ϊ255mg��
=306mg��385mg�������н���ʣ�࣬����㣬
��1���ҡ�����������ȫ�����Ը��ݷ�Ӧ�����������������������ʵ���Ũ�ȣ�������ȫ��Ӧ��������336mL�����������ʵ���Ϊ
=0.015mol��������Ԫ���غ��֪n��HCl��=2n��H2��=2��0.015mol=0.03mol������������ʵ���Ũ��Ϊ
=1mol/L��
�ʴ�Ϊ��1mol/L��
��2������������ʣ�࣬������ȫ��Ӧ����ʱ��������280mL���ʿ��Ը��ݼ������ݼ�����������ʵ���֮�ȣ���þ���������ʵ����ֱ�Ϊxmol��ymol�����ݶ���������֪24x+27y=0.255�����ݵ���ת���غ���2x+3y=
��2���������̽�ã�x=0.005��y=0.005���ʺϽ���þ���������ʵ���֮��Ϊ0.005mol��0.005mol=1��1��
�ʴ�Ϊ��1��1��
��3������ʵ����������м���һ������NaOH��Һ��ǡ��ʹ��Ԫ��ȫ����AlO2-��ʽ���ڣ���ʹMg2+�պó�����ȫ����ʱ��Һ������ΪNaCl��NaAlO2�������������غ㣬��n��NaOH��=n��NaCl��+n��NaAlO2�������Cl�����ӡ�Alԭ���غ㣬��֪n��NaOH��=n��NaCl��+n��NaAlO2��=n��HCl��+n��Al��=0.03mol+0.005mol=0.035mol������Ҫ1mol/L NaOH��Һ�����Ϊ
=0.035L��
�ʴ�Ϊ��0.035��
��4����ʼ���ܷ�����Ӧ��2NaOH+CO2=Na2CO3+H2O��Ȼ����������ķ�ӦΪ��2AlO2-+CO2+3H2O=2Al��OH��3��+CO32-��ͨ��CO2����2.24Lʱ������11.7�˵ij���������Ϊ���������������ʵ���=
=0.15��������̼���ʵ���Ϊ0.1mol�������ɳ���ʱ���Ķ�����̼Ϊ0.075mol������NaOH��Ӧ�Ķ�����̼Ϊ0.1mol-0.075mol=0.025mol����ʣ����������Ϊ0.025mol��2=0.05mol��
ͨ��CO2����1.12Lʱ�������ʵ���Ϊ
=0.05mol����
2NaOH+CO2=Na2CO3+H2O
0.05mol 0.025mol
��ʣ��0.025mol������̼��ƫ�����Ʒ�Ӧ����������
2AlO2-+CO2+3H2O=2Al��OH��3��+CO32-��
0.025mol 0.05mol
�ʲ�������������=0.05mol��78g/mol=3.9g
�����ɵij�����3.9�ˣ�
| 336mL |
| 280mL |
��1���ҡ�����������ȫ�����Ը��ݷ�Ӧ�����������������������ʵ���Ũ�ȣ�������ȫ��Ӧ��������336mL�����������ʵ���Ϊ
| 0.336L |
| 22.4L/mol |
| 0.03mol |
| 0.03L |
�ʴ�Ϊ��1mol/L��
��2������������ʣ�࣬������ȫ��Ӧ����ʱ��������280mL���ʿ��Ը��ݼ������ݼ�����������ʵ���֮�ȣ���þ���������ʵ����ֱ�Ϊxmol��ymol�����ݶ���������֪24x+27y=0.255�����ݵ���ת���غ���2x+3y=
| 0.28L |
| 22.4L/mol |
�ʴ�Ϊ��1��1��
��3������ʵ����������м���һ������NaOH��Һ��ǡ��ʹ��Ԫ��ȫ����AlO2-��ʽ���ڣ���ʹMg2+�պó�����ȫ����ʱ��Һ������ΪNaCl��NaAlO2�������������غ㣬��n��NaOH��=n��NaCl��+n��NaAlO2�������Cl�����ӡ�Alԭ���غ㣬��֪n��NaOH��=n��NaCl��+n��NaAlO2��=n��HCl��+n��Al��=0.03mol+0.005mol=0.035mol������Ҫ1mol/L NaOH��Һ�����Ϊ
| 0.035mol |
| 1mol/L |
�ʴ�Ϊ��0.035��
��4����ʼ���ܷ�����Ӧ��2NaOH+CO2=Na2CO3+H2O��Ȼ����������ķ�ӦΪ��2AlO2-+CO2+3H2O=2Al��OH��3��+CO32-��ͨ��CO2����2.24Lʱ������11.7�˵ij���������Ϊ���������������ʵ���=
| 11.7g |
| 78g/mol |
ͨ��CO2����1.12Lʱ�������ʵ���Ϊ
| 1.12L |
| 22.4L/mol |
2NaOH+CO2=Na2CO3+H2O
0.05mol 0.025mol
��ʣ��0.025mol������̼��ƫ�����Ʒ�Ӧ����������
2AlO2-+CO2+3H2O=2Al��OH��3��+CO32-��
0.025mol 0.05mol
�ʲ�������������=0.05mol��78g/mol=3.9g
�����ɵij�����3.9�ˣ�
���������⿼������ļ��㣬���ݱ������ݹ�ϵ�жϷ�Ӧ�Ĺ��������ǹؼ�����4����ע�����ù��̷��ж�ʣ���������ƣ���Ŀ�Ѷ��еȣ����ؿ���ѧ�������ݵķ���������������������Ŀ��飮

��ϰ��ϵ�д�
�����Ŀ
��һ�������£�A��B��Ӧ������C��D���������仯���£�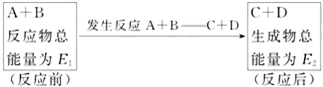
�����йط�ӦA+B�TC+D��˵����ȷ���ǣ�������
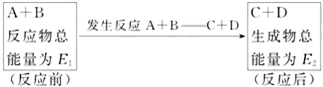
�����йط�ӦA+B�TC+D��˵����ȷ���ǣ�������
| A����Ӧǰ��ԭ�ӵ��������Ŀ���ܸı� |
| B���÷�Ӧ���������仯����һ����������ԭ��Ӧ |
| C�����÷�ӦΪ���ȷ�Ӧ��������ȷ�Ӧ��һ���ܽ��� |
| D���÷�Ӧ��ѭ�����غ㣬����һ���������仯 |
ij��Һ�к���HCO3-��SO32-��CO32-��Cl-���������ӣ������м���������Na2O2�������Һ������Ũ�ȱ��ֲ�����ǣ�������Һ����ޱ仯����������
| A��HCO3- |
| B��SO32- |
| C��CO32- |
| D��Cl- |
����˵����ȷ���ǣ�������
| A��Ħ�����������ĵ�λ |
| B�������£�1mol NH3������С��17g |
| C��������Ħ��������4g/mol |
| D��1mol������ռ�����ԼΪ22.4L |
�������ʷ����仯ʱ�����˷������Ӽ��������ͬ�����͵��ǣ�������
| A������ɱ��ֱ����ȱ�Ϊ���� |
| B�������������ֱ����ȱ�Ϊ���� |
| C���Ȼ������Ȼ���ֱ��ܽ���ˮ�� |
| D���������辧������ֱ������ۻ� |
���������л�Ϊͬ���칹����ǣ�������
| A����������� |
| B��������̼�ɱ� |
| C��ʯī�ͽ��ʯ |
| D����������춡�� |
