��Ŀ����
1����ʯ����Ҫ�ɷ�ΪCaC2������ΪCa3P2��CaS�ȣ�����Ҫ�Ļ�������ԭ�ϣ���Ҫ����������Ȳ������1��Ϊ̽��̼���ƺϳɻ�������ѧ����Ϊ���ܴ�������5�ַ�Ӧ��״̬��ʡ�ԣ���
��CaO+3C?CaC2+COƽ�ⳣ��K1
��CaO+C?Ca+COƽ�ⳣ��K2
��Ca+2C?CaC2ƽ�ⳣ��K3
��2CaO+CaC2?3Ca+2COƽ�ⳣ��K4
��CaC2?Ca+2Cƽ�ⳣ��K5
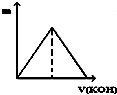
��Ӧͼ�����£�
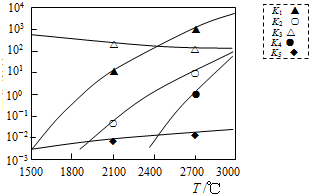
���������ڷ��ȷ�Ӧ���Ǣۣ�����ţ�����ƽ�ⳣ������2700�����Ϸ�Ӧ������С���Ǣݢݣ�����ţ���
����֪2000��ʱ����CaO��s��+C��s��?Ca��g��+CO��g����H1=a kJ•mol-1 ��Ca��g��+2C��s��?CaC2��s����H2=b kJ•mol-1
��2CaO��s��+CaC2��s��?3Ca��g��+2CO��g����H3
���H3=2a-bkJ•mol-1���ú�a��b�Ĵ���ʽ��ʾ��
��2��Ŀǰ��ҵ�Ϻϳɵ�ʯ��Ҫ�������ȷ���
��֪��CaO��s��+3C��s��=CaC2��s��+CO��g����H=+464.1kJ•mol-1
C��s��+$\frac{1}{2}$O2��g��=CO��g����H=-110.5kJ•mol-1
��������������ɢ������ת���ʾ�Ϊ100%������¯�г���������ֻ��CO����Ϊ��ά����ƽ�⣬ÿ����1molCaC2����Ͷ�ϵ���Ϊ��1molCaO��7.2molC ��2.1molO2��
��3������Ȳ��Ĺ��������Ҫ�ɷ�ΪCa��OH��2����������ȡƯ�ۣ�����ȡƯ�۵Ļ�ѧ����ʽΪ2Ca��OH��2+2Cl2=CaCl2+Ca��ClO��2+2H2O������Ȳ����������֮һPH3������������ȩ��������70�桢Al��OH��3���������£��ɺϳ�THPC��ȼ��{[P��CH2OH��4]Cl }���÷�Ӧ�Ļ�ѧ����ʽΪPH3+4HCHO+HCl$\frac{\underline{\;��������\;}}{70��}$[P��CH2OH��4]Cl��
���� ��1�����¶�Խ��KֵԽС�ķ�Ӧ�Ƿ��ȷ�Ӧ����Ӧ������С����ƽ�ⳣ��������ƽ̹���ɴ˷������
���ɷ�Ӧ����CaO��s��+C��s��?Ca��g��+CO��g����H1=a kJ•mol-1����Ca��g��+2C��s��?CaC2��s����H2=b kJ•mol-1�����ݸ�˹���ɣ�Ŀ�귴Ӧ�ķ�Ӧ��Ϊ���ڡ�2-�۵ã�
��2����������������ɢ������ת���ʾ�Ϊ100%������¯�г���������ֻ��CO����Ϊ��ά����ƽ�⣬����ÿ����1molCaC2����Ͷ�ϵ���Ϊ��1molCaO����Ͷ��̼����Ϊ��3mol+$\frac{464.1kJ}{110.5kJ•mo{l}^{-1}}$=7.2mol�����������ʵ���Ϊ��$\frac{464.1kJ}{110.5kJ•mo{l}^{-1}}$��$\frac{1}{2}$=2.1mol��
��3������Ȳ��Ĺ��������Ҫ�ɷ�ΪCa��OH��2����������ȡƯ�ۣ�����ȡƯ�۵Ļ�ѧ����ʽΪ2Ca��OH��2+2Cl2=CaCl2+Ca��ClO��2+2H2O������Ȳ����������֮һPH3������������ȩ��������70�桢Al��OH��3���������£��ɺϳ�THPC��ȼ��{[P��CH2OH��4]Cl }���÷�Ӧ�Ļ�ѧ����ʽΪPH3+4 HCHO+HCl $\frac{\underline{\;��������\;}}{70��}$[P��CH2OH��4]Cl��
��� �⣺��1�����¶�Խ��KֵԽС�������Ǣۣ����Ԣ��Ƿ��ȷ�Ӧ����Ӧ������С����ƽ�ⳣ��������ƽ̹�Ǣݣ��ʴ�Ϊ���ۣ��ݣ���2�֣���
���ɷ�Ӧ����CaO��s��+C��s��?Ca��g��+CO��g����H1=a kJ•mol-1����Ca��g��+2C��s��?CaC2��s����H2=b kJ•mol-1�����ݸ�˹���ɣ�Ŀ�귴Ӧ�ķ�Ӧ��Ϊ���ڡ�2-�۵á�H3=��2a-b��kJ•mol-1���ʴ�Ϊ��2a-b��
��2����������������ɢ������ת���ʾ�Ϊ100%������¯�г���������ֻ��CO����Ϊ��ά����ƽ�⣬����ÿ����1molCaC2����Ͷ�ϵ���Ϊ��1molCaO����Ͷ��̼����Ϊ��3mol+$\frac{464.1kJ}{110.5kJ•mo{l}^{-1}}$=7.2mol�����������ʵ���Ϊ��$\frac{464.1kJ}{110.5kJ•mo{l}^{-1}}$��$\frac{1}{2}$=2.1mol���ʴ�Ϊ��7.2��2.1��
��3������Ȳ��Ĺ��������Ҫ�ɷ�ΪCa��OH��2����������ȡƯ�ۣ�����ȡƯ�۵Ļ�ѧ����ʽΪ2Ca��OH��2+2Cl2=CaCl2+Ca��ClO��2+2H2O������Ȳ����������֮һPH3������������ȩ��������70�桢Al��OH��3���������£��ɺϳ�THPC��ȼ��{[P��CH2OH��4]Cl }���÷�Ӧ�Ļ�ѧ����ʽΪPH3+4 HCHO+HCl $\frac{\underline{\;��������\;}}{70��}$[P��CH2OH��4]Cl���ʴ�Ϊ��2Ca��OH��2+2Cl2=CaCl2+Ca��ClO��2+2H2O��PH3+4 HCHO+HCl $\frac{\underline{\;��������\;}}{70��}$[P��CH2OH��4]Cl��
���� ���⿼��ѧ����ѧƽ�ⳣ������˹�����ԡ���ѧƽ���ƶ���Ӱ��ͻ�ѧ����ʽ����д�����֪ʶ���ۺ���ǿ���ѶȲ���

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�| A�� | ����ɫ��Һ�У�NH4+��Fe2+��SO42-��CO32- | |
| B�� | �ں�����Ba2+��Һ�У�NH4+��Na+��Cl-��OH- | |
| C�� | ��ǿ����Һ�У�Na+��K+��Cl-��SO32- | |
| D�� | �����Ե���Һ�У�K+��Fe2+��Cl-��CH3COO- |
| A�� | ��ԭ���� | B�� | ���� | C�� | ԭ���� | D�� | ��� |
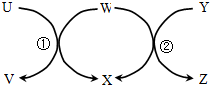 ��ͼ��U��Z�����������ʵ���������ʵ��ͼʾ��ͷ����һ��ת�����ҷ�Ӧ�١��ھ�Ϊ�û���Ӧ�������������������ǣ�������
��ͼ��U��Z�����������ʵ���������ʵ��ͼʾ��ͷ����һ��ת�����ҷ�Ӧ�١��ھ�Ϊ�û���Ӧ�������������������ǣ�������| ��� | U | W | Y | X |
| �� | Na | H2O | Na2O2 | NaOH |
| �� | Fe | H2O | C | H2 |
| �� | HBr | Cl2 | CH4 | HCl |
| �� | CuCl2��aq�� | Al | HCl��aq�� | AlCl3��aq�� |
| A�� | �ڢ� | B�� | �ڢ� | C�� | �٢ڢ� | D�� | �٢ڢۢ� |
��1����ˮ������������Եõ���ˮ�Ȼ�þ����ˮ�Ȼ�þ�ǹ�ҵ��ȡþ��ԭ�ϣ���д������ˮ�Ȼ�þ��ȡ����þ�Ļ�ѧ��Ӧ����ʽMgCl2 $\frac{\underline{\;���\;}}{\;}$Mg+Cl2����
��2��ij���������������л��������Cu2+��Pb2+����ˮ���ŷ�ǰ���ó�������ȥ���������ӣ������������ݣ�����ΪͶ��Na2S��ѡ�Na2S����NaOH����Ч�����ã�
| ���ܵ���� | Cu��OH��2 | CuS | Pb��OH��2 | PbS |
| Ksp | 4.8��10-20 | 6.3��10-36 | 1.2��10-15 | 1.0��10-28 |
����Ȼ��ˮ��pH��8��ˮ����Ҫ����Na+��K+��Ca2+��Mg2+��Cl-��SO42-��Br-��CO32-��HCO3-�����ӣ��������ӷ���ʽ������Ȼ��ˮ�������Ե�ԭ��CO32-+H2O?HCO3-+OH-�� HCO3-+H2O?H2CO3+OH-����дһ������
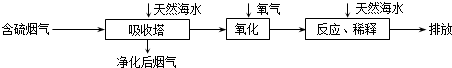
��ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ����������Ȼ��ˮ���պ���������ģ��ʵ�飬ʵ������ͼ��ʾ��

�������ͼʾʵ��������������һ��Ũ�Ⱥ���������SO2������Ч�ʣ����һ�����������飺���ͺ��������¶ȣ������٣���
����Ȼ��ˮ�����˺��������������H2SO3��HSO3-�ȷ��ӻ����ӣ�ͨ�����������Ļ�ѧԭ����2H2SO3+O2=4H++2SO42-��2HSO3-+O2=2H++2SO42-����дһ����ѧ����ʽ�����ӷ���ʽ����������ġ���ˮ����Ҫ�����������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ�����к͡�ϡ�;�����������ˮ�����ɵ��ᣨH+����
| A�� | �ȵĴ�����Һ����Ĵ�����Һȥ����Ч���� | |
| B�� | ʢ��Na2CO3��Һ���Լ�ƿ���������� | |
| C�� | ��AlCl3��Һ���ȡ����ɡ����գ��ɵõ�����Al2O3 | |
| D�� | ���ڳ�ʪ�Ļ���������������ˮ���� |
| A�� | �Ʊ�����ϸ��ƿ�в���ú��Һ�� | |
| B�� | ������ˮ��������ɫƿ�в������䰵�� | |
| C�� | NaOH��Һ��������ɫ�Լ�ƿ�в�Ҫ�������� | |
| D�� | ����������Һ����ڼ����������۵��Լ�ƿ�� |