��Ŀ����
��Դ���������Ϊ��Լ���÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����

��1��̫������ˮ���г�ʹ��һ�����������Ͻ������Ϊ���ռ���̫��������Ϳ�㣬д����̬��ԭ�ӵĺ�������Ų�ʽ ��
��2������ϩ���������ھ������õĹ�����ܣ�����ϩ��C60���Ľṹ��ͼ1��������̼ԭ�ӹ�����ӻ�����Ϊ ��1mol C60�����ЦҼ�����ĿΪ ����ѧ�Ұ�C60��K������һ��������Ļ�������г������ܣ��侧����ͼ2��ʾ���û������е�Kԭ�Ӻ�C60���ӵĸ�����Ϊ ��
��3����Ԫ�����ﱡĤ̫���ܵ�ز���Ϊ���Σ�����Ҫ�����黯�ء����ӡ��ڻ��Ӽ�ͭ������Ĥ��صȣ��ٵ�һ�����ܣ�As Se�����������������=������
�ڶ����������ӵĿռ乹��Ϊ ��
��4������̪ݼ������Ӧ���ڹ�̫���ܵ���У�һ�ֽ���þ̪ݼ�����Ľṹ��ͼ3������ͼ���ü�ͷ��ʾ����λ����λ�� ��

��1��̫������ˮ���г�ʹ��һ�����������Ͻ������Ϊ���ռ���̫��������Ϳ�㣬д����̬��ԭ�ӵĺ�������Ų�ʽ
��2������ϩ���������ھ������õĹ�����ܣ�����ϩ��C60���Ľṹ��ͼ1��������̼ԭ�ӹ�����ӻ�����Ϊ
��3����Ԫ�����ﱡĤ̫���ܵ�ز���Ϊ���Σ�����Ҫ�����黯�ء����ӡ��ڻ��Ӽ�ͭ������Ĥ��صȣ��ٵ�һ�����ܣ�As
�ڶ����������ӵĿռ乹��Ϊ
��4������̪ݼ������Ӧ���ڹ�̫���ܵ���У�һ�ֽ���þ̪ݼ�����Ľṹ��ͼ3������ͼ���ü�ͷ��ʾ����λ����λ��
���㣺ԭ�Ӻ�������Ų�,���ۼ����γɼ����ۼ�����Ҫ����,�жϼ��ӻ����ӵĹ���,�����ļ���
ר�⣺��ѧ���뾧��ṹ
��������1����ԭ�Ӻ�����28�����ӣ����ݹ���ԭ����д���̬��ԭ�ӵĺ�������Ų�ʽ��
��2������ÿ��̼ԭ�Ӻ��еĦҼ�����ȷ�����ӻ���ʽ�����þ�̯������Ҽ����������ݾ�̯�����㾧����Kԭ�ӡ�C60������Ŀ��ȷ����Ŀ֮�ȣ�
��3����ͬһ����Ԫ�صĵ�һ����������ԭ�����������������������ƣ���ע���VA��Ԫ�ش�������Ԫ�صĵ�һ�����ܣ�
�ڼ���Seԭ�Ӽ۲���Ӷԡ��µ��Ӷԣ�ȷ����ռ乹�ͣ�
��4����λ�����ṩ�µ��ӶԵ�ԭ��ָ���ṩ�չ����ԭ�ӣ�
��2������ÿ��̼ԭ�Ӻ��еĦҼ�����ȷ�����ӻ���ʽ�����þ�̯������Ҽ����������ݾ�̯�����㾧����Kԭ�ӡ�C60������Ŀ��ȷ����Ŀ֮�ȣ�
��3����ͬһ����Ԫ�صĵ�һ����������ԭ�����������������������ƣ���ע���VA��Ԫ�ش�������Ԫ�صĵ�һ�����ܣ�
�ڼ���Seԭ�Ӽ۲���Ӷԡ��µ��Ӷԣ�ȷ����ռ乹�ͣ�
��4����λ�����ṩ�µ��ӶԵ�ԭ��ָ���ṩ�չ����ԭ�ӣ�
���
�⣺��1����ԭ�Ӻ�����28�����ӣ����̬��ԭ�ӵĺ�������Ų�ʽΪ��1s22s22p63s23p63d84s2��
�ʴ�Ϊ��1s22s22p63s23p63d84s2��
��2��ÿ��̼ԭ���γ�3���Ҽ������Ҳ����µ��Ӷԣ����Բ���sp2 �ӻ���ÿ��̼ԭ�Ӻ��еĦҼ�����Ϊ
������1mol C60�����ЦҼ�����Ŀ=1mol��60��
��NAmol-1=90NA��
������Kԭ����Ŀ=2��6��
=6��C60������Ŀ=1+8��
=2���ʾ�����Kԭ�ӡ�C60������Ŀ֮��=6��2=3��1��
�ʴ�Ϊ��sp2��90NA��3��1��
��3����As��Se����ͬһ���ڣ���As���ڵ�VA�壬Se���ڵ�VIA�壬Asԭ��4p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���Se��
�ʴ�Ϊ������
�ڶ�������������Seԭ�ӹµ��Ӷ���=
=1���۲���Ӷ�=2+1=3��������ռ�ṹΪV�Σ�
�ʴ�Ϊ��V�Σ�
��4����λ�����ṩ�µ��ӶԵ�ԭ��ָ���ṩ�չ����ԭ�ӣ����Ը�������е���λ��Ϊ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
�ʴ�Ϊ��1s22s22p63s23p63d84s2��
��2��ÿ��̼ԭ���γ�3���Ҽ������Ҳ����µ��Ӷԣ����Բ���sp2 �ӻ���ÿ��̼ԭ�Ӻ��еĦҼ�����Ϊ
| 3 |
| 2 |
| 3 |
| 2 |
������Kԭ����Ŀ=2��6��
| 1 |
| 2 |
| 1 |
| 8 |
�ʴ�Ϊ��sp2��90NA��3��1��
��3����As��Se����ͬһ���ڣ���As���ڵ�VA�壬Se���ڵ�VIA�壬Asԭ��4p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���Se��
�ʴ�Ϊ������
�ڶ�������������Seԭ�ӹµ��Ӷ���=
| 6-2��2 |
| 2 |
�ʴ�Ϊ��V�Σ�
��4����λ�����ṩ�µ��ӶԵ�ԭ��ָ���ṩ�չ����ԭ�ӣ����Ը�������е���λ��Ϊ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
�����������Ƕ����ʽṹ�Ŀ��飬�漰��������Ų�ʽ���ӻ��������ѧ���������ܡ��������������֪ʶ�㣬�����þ�̯�����о����йؼ��㣬ע����λ���γ�������

��ϰ��ϵ�д�
�����Ŀ
���и�����������Һ������������ԭ��Ӧ�����ܴ���������ǣ�������
| A��H+��NO3-��Fe2+��Na+ |
| B��K+��Ba2+��OH-��SO42- |
| C��Fe2+��NO3-��I-��K+ |
| D��Cu2+��NH4+��Cl-��OH- |
X��Y��Z��R��W�����ֶ�����Ԫ�أ�ԭ�����������������ǿ�������ӻ�����Z2Y���ۻ�����RY3��XW4����֪Y��Rͬ���壬Z��R��Wͬ���ڣ�����˵��������ǣ�������
| A��ԭ�Ӱ뾶��Z��R��W |
| B����̬�⻯���ȶ��ԣ�HW��H2R |
| C��XW4�����и�ԭ�Ӿ�����8���ӽṹ |
| D��Y��Z��R����Ԫ����ɵĻ�����ˮ��Һһ���Լ��� |
����˵����ȷ���ǣ�������
| A��0.1 mol/L��������Һ����NH4Al��SO4��2 ��NH4Cl ��NH3?H2O ��CH3COONH4��c��NH4+���ɴ�С��˳���ǣ��ڣ��٣��ܣ��� |
| B��NaHCO3��Һ��Na2CO3��Һ���һ�����ڣ�c��Na+��+c��H+��=c��OH-��+c��HCO3-��+c��CO32-�� |
| C��25��ʱ��pH=11�İ�ˮ��pH=3������������ϣ�c��NH4+����c��SO42-����c��OH-����c��H+�� |
| D�������£���100mL 0.1mol/L NH4HSO4��Һ�еμ�0.1mol/L NaOH��Һ�����ԣ������Һ�и�����Ũ�ȴ�С��ϵ��c��Na+����c��NH4+����c��SO42-����c��OH-��=c��H+�� |
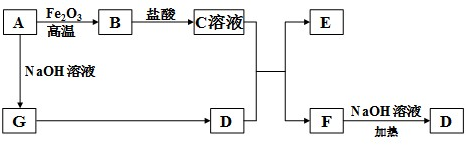
 20.00mLһ�����ʵ���Ũ�ȵ�����X����һ��Ũ�ȵ�NaOH��ҺY�ζ����ζ���������ҺpH������NaOH��Һ������Ĺ�ϵ��ͼ��ʾ��������仯���Բ��ƣ�
20.00mLһ�����ʵ���Ũ�ȵ�����X����һ��Ũ�ȵ�NaOH��ҺY�ζ����ζ���������ҺpH������NaOH��Һ������Ĺ�ϵ��ͼ��ʾ��������仯���Բ��ƣ� ���÷�Ӧ��Cu2Cl2+C2H2+2NH3��Cu2C2����Ȳ��ͭ����ɫ��+2NH4Cl�ɼ�����Ȳ��
���÷�Ӧ��Cu2Cl2+C2H2+2NH3��Cu2C2����Ȳ��ͭ����ɫ��+2NH4Cl�ɼ�����Ȳ��