��Ŀ����
14����֪A��B��C��D��E��FΪԪ�����ڱ���ǰ��������ԭ�������������������Ԫ�أ�����A��B��C��D�˵����֮��Ϊ36��A��Cԭ�ӵ�����������֮�͵���Bԭ�ӵĴ���� ��������Dԭ��������ΪBԭ����������������EԪ�����������Ϊ������F�ļ۵����� ��C�ĺ˵������ȣ���1�����й�����������Ԫ�ص�˵����ȷ����c��d��
a��B��C��D��ԭ�Ӱ뾶�ɴ�С��˳��Ϊ��D��C��B
b��E��F���������������
c��A��B��C��D����Ԫ���е縺�Ժ͵�һ���������ľ�ΪB
d��B��C�γɵĻ������п��ܺ��зǼ��Լ�
e��A��C��Fλ�����ڱ���3��
��2��B����������ͬ�������壮������ˮ���ܽ�Ƚϴ����O3���ѧʽ����ԭ����O3Ϊ���Է��ӣ���O2Ϊ�Ǽ��Է��ӣ�����������ԭ��֪O3�����ܽ��ڼ����ܼ�ˮ�У�
��3��EA2��A2B�۵�ϸߵ���CaH2���ѧʽ����ԭ����CaH2Ϊ���Ӿ��壬H2OΪ���Ӿ��壮
��4��D��B�����γ����ַ��ӣ�����DB2��������ԭ�ӵ��ӻ�������sp2��
���з��ӻ���������DB3�ṹ���Ƶ���c��
a��NH3 b��SO32-c��NO3-d��PCl3
��5����֪B��F���γ����ֻ�����侧����ͼ��ʾ�������ʱ����ת��Ϊ�ҵ�ԭ��ΪCu2O��Cu��3d���Ϊ3d10ȫ����״̬���Ƚ��ȶ���
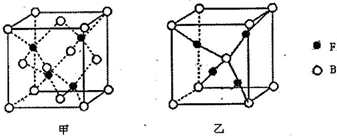
���Ҿ����ܶ�Ϊpg/cm3�����Ҿ����ľ�������Ϊa=$\root{3}{\frac{288}{p{N}_{A}}}$��109 nm��
���� A��B��C��D��E��FΪԪ�����ڱ���ǰ��������ԭ�������������������Ԫ�أ�A��B��C��D�˵����֮��Ϊ36����B����λ�ڵ������ڣ����ڵ���������Na��Mg��Al����ԭ������֮����СΪ36��A��Cԭ�ӵ�����������֮�͵���Bԭ�ӵĴ�������������B���ڵڶ����ڣ�A��Cԭ��������������Ϊ1�����ߴ���IA�壬C��D�����ܴ��ڵ������ڣ���Ϊ����������K��Ca����ԭ������֮����СΪ39����CҲ���ڶ�����Ԫ�أ�C��ԭ�������ִ���B����CΪNa��AΪ���ﮣ�A��B��Dԭ������֮��Ϊ36-11=25��Dԭ��������ΪBԭ�����ӵ���������AΪHԪ�ء�BΪOԪ�ء�DΪSԪ�أ�EԪ�����������Ϊ��������E���ڢ�A�壬ԭ������������EΪCa�����Ԫ�����������������ʽ��
��� �⣺A��B��C��D��E��FΪԪ�����ڱ���ǰ��������ԭ�������������������Ԫ�أ�A��B��C��D�˵����֮��Ϊ36����B����λ�ڵ������ڣ����ڵ���������Na��Mg��Al����ԭ������֮����СΪ36��A��Cԭ�ӵ�����������֮�͵���Bԭ�ӵĴ�������������B���ڵڶ����ڣ�A��Cԭ��������������Ϊ1�����ߴ���IA�壬C��D�����ܴ��ڵ������ڣ���Ϊ����������K��Ca����ԭ������֮����СΪ39����CҲ���ڶ�����Ԫ�أ�C��ԭ�������ִ���B����CΪNa��AΪ���ﮣ�A��B��Dԭ������֮��Ϊ36-11=25��Dԭ��������ΪBԭ�����ӵ���������AΪHԪ�ء�BΪOԪ�ء�DΪSԪ�أ�EԪ�����������Ϊ��������E���ڢ�A�壬ԭ������������EΪCa��
��1��a��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶��C��Na����D��S����B��O������a����
b��EΪCa��FΪCu�����������������ֱ�Ϊ2��1����b����
c��H��O��Na��S����Ԫ���е縺�Ժ͵�һ���������ľ�ΪO����c��ȷ��
d��B��C�γɵĻ�����Ϊ�����ơ��������ƣ����������к��зǼ��Լ�����d��ȷ��
e��H��Naλ�����ڱ���s����Cu�������ڱ���ds������e����
�ʴ�Ϊ��cd��
��2��B����������ͬ����������������������O2����Ϊ�Ǽ��Է��ӣ���O3����Ϊ���Է��ӣ�ˮ�����Ǽ��Է��ӣ��������ܣ�����ˮ���ܽ�Ƚϴ����O3��
�ʴ�Ϊ��O3��O3Ϊ���Է��ӣ���O2Ϊ�Ǽ��Է��ӣ�����������ԭ��֪O3�����ܽ��ڼ����ܼ�ˮ�У�
��3��CaH2�������Ӿ��壬H2O���ڷ��Ӿ��壬�۵�ϸߵ���CaH2��
�ʴ�Ϊ��CaH2��CaH2�������Ӿ��壬H2O���ڷ��Ӿ��壻
��4��SO2������Sԭ�ӹµ��Ӷ���=$\frac{6-2��2}{2}$=1���۲���Ӷ���=2+1=3����Sԭ�ӵ��ӻ������� sp2��
SO3��Sԭ�ӹµ��Ӷ���=$\frac{6-2��3}{2}$=0���۲���Ӷ���Ϊ3+3=3��Ϊƽ�������νṹ��NH3Ϊ�����νṹ��SO32-��Sԭ�ӹµ��Ӷ���=$\frac{6+2-2��3}{2}$=1���۲���Ӷ���=3+1=4��Ϊ�����νṹ��NO3- ��Nԭ�ӹµ��Ӷ���=$\frac{5+1-2��3}{2}$=0���۲���Ӷ���=3+0=3��Ϊƽ�������νṹ��PCl3Ϊ�����νṹ������NO3-�Ľṹ��SO3���ƣ�
�ʴ�Ϊ��sp2��c��
��5������Cuԭ����ĿΪ4����ԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4����ѧʽΪCuO������Cuԭ����ĿΪ4����ԭ����ĿΪ1+8��$\frac{1}{8}$=2����ѧʽΪCu2O��Cu2+��Χ�����Ų�Ϊ3d9����Cu+��Χ�����Ų�Ϊ3d10�������ȶ��ṹ���ʸ�����CuO����ת��ΪCu2O��
�Ҿ����ܶ�Ϊpg/cm3���Ҿ�������Ϊ2��$\frac{144}{{N}_{A}}$g��������=$\root{3}{\frac{2��\frac{144}{{N}_{A}}g}{pg/c{m}^{3}}}$cm=$\root{3}{\frac{288}{p{N}_{A}}}$cm=$\root{3}{\frac{288}{p{N}_{A}}}$��109 nm��
�ʴ�Ϊ��CuO��Cu2+��Χ�����Ų�Ϊ3d9����Cu2O��Cu+��Χ�����Ų�Ϊ3d10�������ȶ��ṹ��$\root{3}{\frac{288}{p{N}_{A}}}$��109 nm��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�ƶ�Ԫ���ǽ���ؼ����漰��������Ų���Ԫ�������ɡ������ܡ��縺�ԡ��ӻ���ʽ��ռ乹���жϡ����ӽṹ�����ʡ���������ȣ�Ԫ���ƶ��ѶȽϴϺõؿ���ѧ�����������������ѶȽϴ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� Ŀ�����ϵ�д�
Ŀ�����ϵ�д� 1Lij�����Һ�����ܺ��е����������
1Lij�����Һ�����ܺ��е����������| ���ܴ������е������� | H+��NH4+��Al3+��K+ |
| ���ܴ������е������� | Cl-��Br-��I-��ClO-��AlO2- |
�ٸ���Һ��һ�����е���������H+��NH4+��Al3+��
�ڿ��ܴ��ڵ���������K+��
�ۿ϶������ڵ���������ClO-��AlO2-��
��2������⣬����Һ�л����д�����Cl-��Br-��I-������2L�û����Һ��ͨ��һ������Cl2����Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2���������״�����Ĺ�ϵ�����ʾ��������ش��������⣮
| Cl2���������״���� | 2.8L | 5.6L | 11.2L |
| n��Cl-�� | 1.25mol | 1.5mol | 2mol |
| n��Br-�� | 1.5mol | 1.4mol | 0.9mol |
| n��I-�� | a mol | 0 | 0 |
�ڵ�ͨ��Cl2�����Ϊ3.36L����״̬�£�ʱ����Һ�з�����Ӧ�����ӷ���ʽΪCl2+2I-=2Cl-+I2����ʱ��Һ��Br-��I-�����ʵ���Ũ�ȷֱ�Ϊc��Br-��=0.75mol/L��c��I-��=0.05mol/L��
| A�� | ����p% | B�� | ����p% | ||
| C�� | С��p% | D�� | �ﵽƽ���ʱ���Ҹ��� |
| A�� | �Ҵ������ | B�� | 1-������2-���� | C�� | �����뱽�״� | D�� | ������Ӳ֬�� |
| A�� | N2��H2��һ�������·�Ӧ����NH3 | B�� | ���Ṥ����NH3������NO | ||
| C�� | NH3��HCl����NH4Cl | D�� | ��NH3��̼����狀������ |
| A�� | ���ԣ�HF��HCl��H2S | B�� | �е㣺CBr4��CCl4��CF4 | ||
| C�� | ���ԣ�Ca��OH��2��Mg��OH��2��Al��OH��3 | D�� | �ȶ��ԣ�H2O��H2S��H2Se |
 A��B��C��D��E������Һ�ֱ���NaOH��NH3•H2O��CH3COOH��HCl��NH4HSO4�е�һ�֣������½�������ʵ�飺
A��B��C��D��E������Һ�ֱ���NaOH��NH3•H2O��CH3COOH��HCl��NH4HSO4�е�һ�֣������½�������ʵ�飺