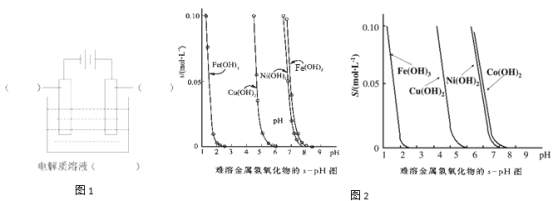
Ő‚ńŅńŕ»›
°ĺŐ‚ńŅ°Ņ“ĹŃ∆…Ō¬Ő∑Į![]() «÷őŃ∆»ĪŐķ–‘∆∂—™ĶńŐō–ß“©£ģń≥ĽĮ—ß–ň»§–°◊ť∂‘¬Ő∑ĮĹÝ––Ńň»ÁŌ¬ĶńŐĹĺŅ£ļ
«÷őŃ∆»ĪŐķ–‘∆∂—™ĶńŐō–ß“©£ģń≥ĽĮ—ß–ň»§–°◊ť∂‘¬Ő∑ĮĹÝ––Ńň»ÁŌ¬ĶńŐĹĺŅ£ļ
ĘŮ![]() ÷∆Īł≤ķ∆∑
÷∆Īł≤ķ∆∑![]()
ł√–°◊ť”…∑ŌŐķ–ľ![]() ļ¨…ŔŃŅ—űĽĮÕ≠°Ę—űĽĮŐķĶ»‘”÷
ļ¨…ŔŃŅ—űĽĮÕ≠°Ę—űĽĮŐķĶ»‘”÷ ![]() £¨”√»ÁÕľňý ĺ◊į÷√÷∆Īł
£¨”√»ÁÕľňý ĺ◊į÷√÷∆Īł![]() ĺßŐŚ£¨≤Ĺ÷Ť»ÁŌ¬£ļ
ĺßŐŚ£¨≤Ĺ÷Ť»ÁŌ¬£ļ
![]() ‘§ī¶ņŪ£ļŌ»Ĺę∑ŌŐķ–ľľ”»ŽĶĹĪ•ļÕ
‘§ī¶ņŪ£ļŌ»Ĺę∑ŌŐķ–ľľ”»ŽĶĹĪ•ļÕ![]() »‹“ļ÷–ŌīĶ”£¨ńŅĶń «______£¨»ĽļůĹę∑ŌŐķ–ľ”√ňģŌīĶ”
»‹“ļ÷–ŌīĶ”£¨ńŅĶń «______£¨»ĽļůĹę∑ŌŐķ–ľ”√ňģŌīĶ”![]() Īť£ģ
Īť£ģ
![]() ĹęŌīĶ”ļůĶń∑ŌŐķ–ľľ”»ŽĶĹ‘≤Ķ◊…’∆Ņ÷–£¨≤Ę≥÷–ÝÕ®»Ž
ĹęŌīĶ”ļůĶń∑ŌŐķ–ľľ”»ŽĶĹ‘≤Ķ◊…’∆Ņ÷–£¨≤Ę≥÷–ÝÕ®»Ž![]() £¨
£¨![]() Ķń◊ų”√ «______£ģ
Ķń◊ų”√ «______£ģ
![]() ‘Ŕľ”»Ž◊„ŃŅŌ°ŃÚňŠ£¨Ņō÷∆ő¬∂»
‘Ŕľ”»Ž◊„ŃŅŌ°ŃÚňŠ£¨Ņō÷∆ő¬∂»![]() ÷ģľš£¨≥š∑÷∑ī”¶ļů£¨‘≤Ķ◊…’∆Ņ÷– £”ŗĶńĻŐŐŚő™______£ģ
÷ģľš£¨≥š∑÷∑ī”¶ļů£¨‘≤Ķ◊…’∆Ņ÷– £”ŗĶńĻŐŐŚő™______£ģ
![]() ĽŮ»°≤ķ∆∑£ļŌ»ŌÚ≤Ĺ÷Ť
ĽŮ»°≤ķ∆∑£ļŌ»ŌÚ≤Ĺ÷Ť![]() ÷–∑ī”¶ļůĶńĽžļŌőÔ÷–ľ”»Ž…Ŕ–Ū’ŰŃůňģ£¨≥√»»Ļż¬ň£¨______
÷–∑ī”¶ļůĶńĽžļŌőÔ÷–ľ”»Ž…Ŕ–Ū’ŰŃůňģ£¨≥√»»Ļż¬ň£¨______![]() ¬ň≥ŲĺßŐŚ£¨”√…ŔŃŅĪýňģŌīĶ”
¬ň≥ŲĺßŐŚ£¨”√…ŔŃŅĪýňģŌīĶ”![]() īő£¨‘Ŕ”√¬ň÷ĹĹęĺßŐŚőŁł…£¨√‹Ī’Ī£īś£ģ
īő£¨‘Ŕ”√¬ň÷ĹĹęĺßŐŚőŁł…£¨√‹Ī’Ī£īś£ģ
ĘÚ![]() ≤‚∂®
≤‚∂®![]() ļ¨ŃŅ
ļ¨ŃŅ![]()
![]() ≥∆»°…Ō Ų—ý∆∑
≥∆»°…Ō Ų—ý∆∑![]() £¨»‹”ŕ ŃŅĶńŌ°ŃÚňŠ÷–£¨Ňš≥…100mL»‹“ļ£¨–Ť“™Ķń“«∆ų≥żŐž∆Ĺ°Ę≤£ŃßįŰ°Ę…’Ī≠°ĘŃŅÕ≤Õ‚£¨ĽĻ–Ť“™Ķń“«∆ų”–
£¨»‹”ŕ ŃŅĶńŌ°ŃÚňŠ÷–£¨Ňš≥…100mL»‹“ļ£¨–Ť“™Ķń“«∆ų≥żŐž∆Ĺ°Ę≤£ŃßįŰ°Ę…’Ī≠°ĘŃŅÕ≤Õ‚£¨ĽĻ–Ť“™Ķń“«∆ų”–![]() ŐÓ“«∆ų√Ż≥∆
ŐÓ“«∆ų√Ż≥∆![]() ______°Ę______£ģ
______°Ę______£ģ
![]() ◊ľ»∑ŃŅ»°25mLł√“ļŐŚ”ŕ◊∂–ő∆Ņ÷–£¨”√
◊ľ»∑ŃŅ»°25mLł√“ļŐŚ”ŕ◊∂–ő∆Ņ÷–£¨”√![]()
![]() ĪÍ◊ľ»‹“ļĶő∂®£¨‘ÚĶő∂®÷’Ķ„ĶńŇ–∂Ō∑Ĺ∑® «______£ģ
ĪÍ◊ľ»‹“ļĶő∂®£¨‘ÚĶő∂®÷’Ķ„ĶńŇ–∂Ō∑Ĺ∑® «______£ģ
![]() ”√Õ¨—ýĶń∑Ĺ∑®Ķő∂®3īő£¨∆ĹĺýŌŻļń
”√Õ¨—ýĶń∑Ĺ∑®Ķő∂®3īő£¨∆ĹĺýŌŻļń![]() ĪÍ◊ľ“ļ£¨ł√—ý∆∑÷–
ĪÍ◊ľ“ļ£¨ł√—ý∆∑÷–![]() Ķń÷ ŃŅ∑÷ żő™______
Ķń÷ ŃŅ∑÷ żő™______![]()
![]() »Ű≤‚ŃŅĹŠĻŻ∆ę–°£¨‘ÚŅ…ń‹ «‘ŕ∂®»› Ī ______
»Ű≤‚ŃŅĹŠĻŻ∆ę–°£¨‘ÚŅ…ń‹ «‘ŕ∂®»› Ī ______ ![]() ŐÓ°įł© ”°ĪĽÚ°į—Ų ”°Ī
ŐÓ°įł© ”°ĪĽÚ°į—Ų ”°Ī![]() ∂Ń ż£ģ
∂Ń ż£ģ

°ĺīūįł°ŅŌī»•Őķ–ľĪŪ√śĶń”ÕőŘ ŇŇ≥ż◊į÷√÷–ĶńŅ’∆ÝĽÚ—ű∆Ý Cu ņš»ī°ĘĹŠĺß 100mL»›ŃŅ∆Ņ ĹļÕ∑ĶőĻ‹ ĶĪ◊Óļů“ĽĶőĪÍ◊ľ“ļĶő»Ž Ī£¨»‹“ļĪšő™◊Ōļž…ę£¨«“30sĪ£≥÷≤ĽĪš ![]() —Ų ”
—Ų ”
°ĺĹ‚őŲ°Ņ
łýĺ›”ÕőŘ‘ŕľÓ–‘ŐűľĢŌ¬“◊ňģĹ‚∑÷őŲĹ‚īū£Ľłýĺ› Ķ—ťńŅĶńľį≤Ŕ◊ųŃų≥Ő∑÷őŲĹ‚īū£Ľłýĺ›ňŠľÓĶő∂®‘≠ņŪľįőÔ÷ ŐŠīŅ”Ž∑÷ņŽ‘≠ņŪ∑÷őŲ Ķ—ťňý–Ť“«∆ų£¨ľįĶő∂® Ī Ķ—ťŌ÷Ōů£Ľłýĺ›Ķő∂® żĺ›ľ∆ň„—ý∆∑ĶńįŔ∑÷ļ¨ŃŅ£Ľłýĺ› Ķ—ť‘≠ņŪĹÝ––őů≤Ó∑÷őŲ°£
![]() ŐľňŠń∆Ķńňģ»‹“ļŌ‘ľÓ–‘£¨Ņ…“‘≥ż»•Őķ–ľĪŪ√śĶń”ÕőŘ£¨ Ļ īūįłő™£ļŌī»•Őķ–ľĪŪ√śĶń”ÕőŘ£Ľ
ŐľňŠń∆Ķńňģ»‹“ļŌ‘ľÓ–‘£¨Ņ…“‘≥ż»•Őķ–ľĪŪ√śĶń”ÕőŘ£¨ Ļ īūįłő™£ļŌī»•Őķ–ľĪŪ√śĶń”ÕőŘ£Ľ
![]() “◊ĪĽ—ű∆Ý—űĽĮ£¨≥÷–ÝÕ®»Ž
“◊ĪĽ—ű∆Ý—űĽĮ£¨≥÷–ÝÕ®»Ž![]() ŇŇ≥ż◊į÷√÷–ĶńŅ’∆ÝĽÚ—ű∆Ý£¨ Ļ īūįłő™£ļŇŇ≥ż◊į÷√÷–ĶńŅ’∆ÝĽÚ—ű∆Ý£Ľ
ŇŇ≥ż◊į÷√÷–ĶńŅ’∆ÝĽÚ—ű∆Ý£¨ Ļ īūįłő™£ļŇŇ≥ż◊į÷√÷–ĶńŅ’∆ÝĽÚ—ű∆Ý£Ľ
![]() —űĽĮÕ≠°Ę—űĽĮŐķ”ŽŃÚňŠ∑ī”¶…ķ≥…ŃÚňŠÕ≠ļÕŃÚňŠŐķ£¨ŐķĶ•÷ ”ŽŃÚňŠÕ≠ļÕŃÚňŠŐķ∑ī”¶…ķ≥…ŃÚňŠ—«ŐķļÕÕ≠Ķ•÷ £¨ Ļ īūįłő™£ļCu£Ľ
—űĽĮÕ≠°Ę—űĽĮŐķ”ŽŃÚňŠ∑ī”¶…ķ≥…ŃÚňŠÕ≠ļÕŃÚňŠŐķ£¨ŐķĶ•÷ ”ŽŃÚňŠÕ≠ļÕŃÚňŠŐķ∑ī”¶…ķ≥…ŃÚňŠ—«ŐķļÕÕ≠Ķ•÷ £¨ Ļ īūįłő™£ļCu£Ľ
≥√»»Ļż¬ňļů£¨Ĺę¬ň“ļņš»īĹŠĺßŅ…Ķ√![]() ĺßŐŚ£¨ Ļ īūįłő™£ļņš»ī°ĘĹŠĺߣĽ
ĺßŐŚ£¨ Ļ īūįłő™£ļņš»ī°ĘĹŠĺߣĽ
![]() ≤Ĺ÷Ťő™£ļľ∆ň„
≤Ĺ÷Ťő™£ļľ∆ň„![]() ≥∆ŃŅ
≥∆ŃŅ![]() »‹“ļ
»‹“ļ![]() ◊™“∆
◊™“∆![]() ŌīĶ”
ŌīĶ”![]() ∂®»›
∂®»›![]() “°‘»
“°‘»![]() ◊į∆Ņ
◊į∆Ņ![]() Őý«©£¨–Ť“™ Ļ”√Ķń“«∆ų”–£ļ100mL»›ŃŅ∆Ņ£¨Õ–ŇŐŐž∆Ĺ°Ę…’Ī≠°Ę≤£ŃßįŰ°ĘĹļÕ∑ĶőĻ‹£¨ŃŅÕ≤Ņ…”√Ņ…≤Ľ”√£¨Ļ īūįłő™£ļ100mL»›ŃŅ∆Ņ£ĽĹļÕ∑ĶőĻ‹£Ľ
Őý«©£¨–Ť“™ Ļ”√Ķń“«∆ų”–£ļ100mL»›ŃŅ∆Ņ£¨Õ–ŇŐŐž∆Ĺ°Ę…’Ī≠°Ę≤£ŃßįŰ°ĘĹļÕ∑ĶőĻ‹£¨ŃŅÕ≤Ņ…”√Ņ…≤Ľ”√£¨Ļ īūįłő™£ļ100mL»›ŃŅ∆Ņ£ĽĹļÕ∑ĶőĻ‹£Ľ
![]() Ņ…ņŻ”√
Ņ…ņŻ”√![]() »‹“ļ◊‘…ŪĶń—’…ę◊ųő™÷ł ĺľŃŇ–∂ŌĶő∂®÷’Ķ„ Ī£¨Ķőľ”
»‹“ļ◊‘…ŪĶń—’…ę◊ųő™÷ł ĺľŃŇ–∂ŌĶő∂®÷’Ķ„ Ī£¨Ķőľ”![]() »‹“ļ Ī£¨»‹“ļĹę”…őř…ęĪšő™◊Ōļž…ę£¨Ļ īūįłő™£ļĶĪ◊Óļů“ĽĶőĪÍ◊ľ“ļĶő»Ž Ī£¨»‹“ļĪšő™◊Ōļž…ę£¨«“30sĪ£≥÷≤ĽĪš£Ľ
»‹“ļ Ī£¨»‹“ļĹę”…őř…ęĪšő™◊Ōļž…ę£¨Ļ īūįłő™£ļĶĪ◊Óļů“ĽĶőĪÍ◊ľ“ļĶő»Ž Ī£¨»‹“ļĪšő™◊Ōļž…ę£¨«“30sĪ£≥÷≤ĽĪš£Ľ
![]() ≤‚∂®—ý∆∑÷–
≤‚∂®—ý∆∑÷–![]() Ķńļ¨ŃŅ£¨≤…”√‘ŕňŠ–‘ŐűľĢŌ¬
Ķńļ¨ŃŅ£¨≤…”√‘ŕňŠ–‘ŐűľĢŌ¬![]() ĪÍ◊ľ“ļĶő∂®£¨
ĪÍ◊ľ“ļĶő∂®£¨![]() £¨Őķ‘™ňōĽĮļŌľŘ…żłŖ1ľŘ£Ľ
£¨Őķ‘™ňōĽĮļŌľŘ…żłŖ1ľŘ£Ľ![]() £¨√Ő‘™ňōĹĶĶÕ5ľŘ£¨ĽĮļŌľŘ…żĹĶ◊Ó–°ĻęĪ∂ żő™5£¨Ļ FŌĶ żő™5£¨
£¨√Ő‘™ňōĹĶĶÕ5ľŘ£¨ĽĮļŌľŘ…żĹĶ◊Ó–°ĻęĪ∂ żő™5£¨Ļ FŌĶ żő™5£¨![]() ŌĶ żő™1£¨łý囑™ňō ōļ„Ņ…÷™
ŌĶ żő™1£¨łý囑™ňō ōļ„Ņ…÷™![]() ”Ž
”Ž![]() ŌĶ ż∑÷Īūő™1°Ę5£¨łýĺ›ĶÁļ… ōļ„Ņ…÷™»ĪŌÓő™
ŌĶ ż∑÷Īūő™1°Ę5£¨łýĺ›ĶÁļ… ōļ„Ņ…÷™»ĪŌÓő™![]() £¨
£¨![]() ∆šŌĶ żő™
∆šŌĶ żő™![]() £¨łýĺ›H‘™ňō ōļ„Ņ…÷™
£¨łýĺ›H‘™ňō ōļ„Ņ…÷™![]() ŌĶ ż «4£¨ňý“‘∑ī”¶ņŽ◊”∑Ĺ≥Ő Ĺő™
ŌĶ ż «4£¨ňý“‘∑ī”¶ņŽ◊”∑Ĺ≥Ő Ĺő™![]() £¨łýĺ›∑Ĺ≥Ő Ĺ÷–ĻōŌĶ Ĺ£ļ
£¨łýĺ›∑Ĺ≥Ő Ĺ÷–ĻōŌĶ Ĺ£ļ ![]() Ķ√£ļ n(Fe2+)=
Ķ√£ļ n(Fe2+)=![]() £¨ňý“‘—ý∆∑÷–
£¨ňý“‘—ý∆∑÷–![]() Ķń÷ ŃŅ
Ķń÷ ŃŅ![]() £¨ňý“‘10
£¨ňý“‘10
≤ķ∆∑÷–![]() Ķń÷ ŃŅ∑÷ żő™
Ķń÷ ŃŅ∑÷ żő™![]() £¨Ļ īūįłő™£ļ
£¨Ļ īūįłő™£ļ![]() £Ľ
£Ľ
![]() ∂®»› Ī—Ų ”ŐŚĽż∆ęīů£¨Ň®∂»∆ę–°£¨Ļ īūįłő™£ļ—Ų ”°£
∂®»› Ī—Ų ”ŐŚĽż∆ęīů£¨Ň®∂»∆ę–°£¨Ļ īūįłő™£ļ—Ų ”°£

 ‘ń∂ŃŅž≥ĶŌĶŃ–īūįł
‘ń∂ŃŅž≥ĶŌĶŃ–īūįł