��Ŀ����
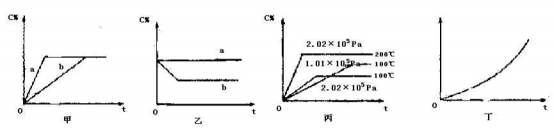
��10�֣�ijͬѧȡһ������Al��Fe������2.0L��ϡ��HNO3��ַ�Ӧ������HNO3�Ļ�ԭ����ȫ��Ϊ��Ρ��ڷ�Ӧ�����Һ�У���μ���4mol��L��1��NaOH��Һ������NaOH��Һ�����������ij��������ʵ����Ĺ�ϵ��ͼ��ʾ������ͼ��ش����⣺

��1��DE�η�����Ӧ�����ӷ���ʽΪ��_____________________________________ ��
��2����д������һ��Al��Fe�뼫ϡHNO3��Ӧ�Ļ�ѧ����ʽ��_______________ ��
��3��B���Ӧ�ij��������ʵ���Ϊ_______mol��C���Ӧ������������Һ�����Ϊ______mL��
��4��ԭ������Һ�����ʵ���Ũ��Ϊ_______mol/L��
(10��,ÿ��2��)(1) NH4++OH��=NH3?H2O��
(2) 8Al+30HNO3=8Al(NO3)3+3NH4NO3+9H2O��8Fe+30HNO3=8Fe(NO3)3+3NH4NO3+9H2O
(3) 0.032�� 7��(4) 0.074��
��������
�����������1��Al��Fe����������ᷴӦ��������Ӧ�����Һ�м���NaOH��ʼ�����γɣ�˵�������������˽��������ᷴӦ����������������������OC�η�����Ӧ��H++ OH -= H2O����CD����Һ�е�Fe3+��Al3+����������Ӧ�γ�Fe(OH)3��Al(OH)3��������DE�Σ����������ʵ���û�б仯������Ϊ��DE��NaOH��Һ�����ᱻ��ԭΪNH4NO3,���߷������ֽⷴӦ�����ӷ���ʽ�ǣ�NH4++ OH-=NH3��H2O����2��8Al+30HNO3=8Al(NO3)3+3NH4NO3+9H2O��8Fe+30HNO3 =8Fe(NO3)3+3NH4NO3+ 9H2O����3����EF��NaOH�ܽ�Al(OH)3���������ӷ���ʽ�ǣ�Al(OH)3+ OH-= AlO2-+ 2H2O���ܽ�Al(OH)3���ĵ�NaOH�����ʵ�����n(NaOH)= 4mol/L�� 0.002L =0.008mol,���Ը��ݷ�Ӧ����ʽ�ж��ߵ����ʵ�����ϵ��֪��n(Al(OH)3)=0.008mol������Al(OH)3�������ĵ�NaOH��Һ�������6ml�����ݷ�Ӧ����ʽNH4++ OH-=NH3��H2O ��֪��n(NH4+)=4mol/L ��0.003L=0.012mol��n(e-)=0.012mol��8=0.096mol,Fe��Al����+3�۵Ľ����������ڷ�Ӧ�����е���ת����Ŀ��ȣ�����n(Fe)+n(Al)= 0.096mol��3=0.032mol,������B���Ӧ�ij��������ʵ�������������ʵ�����ȣ�n(����)= 0.032mol;ʹAl3+��Fe3+�γɳ������ĵ�NaOH�����ʵ�������ӵ����ʵ�����ȣ���0.096mol ����������NaOH��Һ�������V��NaOH��=0.096mol ��4mol/L=0.024L=24ml,���� C���Ӧ������������Һ�����Ϊ31ml-24ml=7ml����4�����ݷ���ʽH++ OH -= H2O��֪n(HNO3)=n(HNO3)(����)+ n(HNO3)(��Ӧ)= 4mol/L��0.007L+0.032mol��(30/8)=0.148mol.����ԭ�����Ũ����c(HNO3)= n(HNO3)��V=0.148mol��2.0L=0.074mol/L.
���㣺����Al��Fe�Ļ�ѧ���ʡ����ӷ���ʽ����д���غ㷨�ڻ�����и��ɷֵ�ȷ����Ӧ�õ�֪ʶ��

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
 xC(g)�������� C�ڷ�Ӧ������еİٷֺ�����C%���ͷ�Ӧʱ�䣨t���Ĺ�ϵ��
xC(g)�������� C�ڷ�Ӧ������еİٷֺ�����C%���ͷ�Ӧʱ�䣨t���Ĺ�ϵ��