��Ŀ����
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���塣B������Ԫ���е縺������Ԫ�ء�B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�6��Ԫ�ء�D��ԭ��������EС4��D��B���γ����ӻ������侧���ṹ��ͼ��

��ش�
��1��AԪ�ص��ʵĵ���ʽ��____________��
��2��B���ʵķ���ʽΪ____________��C��Ԫ�ط�����____________��B��A�γɵĻ������C ��A�γɵĻ�����е�ߣ���ԭ����________________________
��3��E��Ԫ�����ڱ��е�______���ڣ���_______���Ԫ�أ�������____________�����Ļ�̬ԭ�ӵĵ����Ų�ʽΪ____________
��4����ͼ�п��Կ�����D��B�γ������ӻ�������õ���ʽ��ʾ�����ӻ�������γɹ���________________________�������ӻ����ᄃ����ܶ�Ϊag��cm-3�����������____________(ֻҪ���г���ʽ)��
��1��AԪ�ص��ʵĵ���ʽ��____________��
��2��B���ʵķ���ʽΪ____________��C��Ԫ�ط�����____________��B��A�γɵĻ������C ��A�γɵĻ�����е�ߣ���ԭ����________________________
��3��E��Ԫ�����ڱ��е�______���ڣ���_______���Ԫ�أ�������____________�����Ļ�̬ԭ�ӵĵ����Ų�ʽΪ____________
��4����ͼ�п��Կ�����D��B�γ������ӻ�������õ���ʽ��ʾ�����ӻ�������γɹ���________________________�������ӻ����ᄃ����ܶ�Ϊag��cm-3�����������____________(ֻҪ���г���ʽ)��
��1��H��H
��2��F2��HCl����������Ӽ������������Ȼ�����Ӽ�û�����
��3���ģ�VIIB������1s22s22p63s23p63d54s1
��4�� ��
��
��2��F2��HCl����������Ӽ������������Ȼ�����Ӽ�û�����
��3���ģ�VIIB������1s22s22p63s23p63d54s1
��4��
 ��
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ20�ŵ�Ԫ�أ����ǵ�ԭ��������������Aԭ�ӵļ۵��Ӳ��p�����ֻ��1�����ӣ�B��C��DԪ�صĻ�̬ԭ�Ӿ�����ͬ���ܲ�����B��DԪ�ص�ԭ�ӵ�p�ܼ��϶���1��δ�ɶԵ��ӣ�Dԭ�ӵ�һ����������3p�����3p����ѳ�����Cԭ�ӵ�p�������3��δ�ɶԵ��ӣ�E��ͬ���ڵ�һ��������С��Ԫ�أ��ش��������⣺
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ20�ŵ�Ԫ�أ����ǵ�ԭ��������������Aԭ�ӵļ۵��Ӳ��p�����ֻ��1�����ӣ�B��C��DԪ�صĻ�̬ԭ�Ӿ�����ͬ���ܲ�����B��DԪ�ص�ԭ�ӵ�p�ܼ��϶���1��δ�ɶԵ��ӣ�Dԭ�ӵ�һ����������3p�����3p����ѳ�����Cԭ�ӵ�p�������3��δ�ɶԵ��ӣ�E��ͬ���ڵ�һ��������С��Ԫ�أ��ش��������⣺
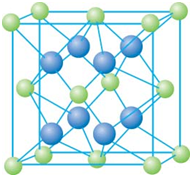 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B�γɵľ����侧���ṹ��ͼ��ͼ��С�����D���������B����ش�
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B�γɵľ����侧���ṹ��ͼ��ͼ��С�����D���������B����ش�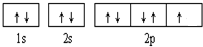
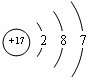
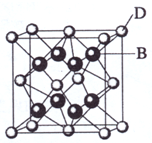 ��2009?���ϣ���֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B���γ����ӻ������侧���ṹ����ͼ��
��2009?���ϣ���֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B���γ����ӻ������侧���ṹ����ͼ��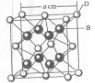 ��2011?����һģ��[��ѧ--ѡ�����ʽṹ������]
��2011?����һģ��[��ѧ--ѡ�����ʽṹ������]