��Ŀ����
17�� ԭ������֮��Ϊ16�����ֶ�����Ԫ��x��y��z��Ӧ�ĵ��� X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ������µ���X��Y��Z֮����Է�����ͼ��ʾ�ı仯����֪B���������zԭ�Ӹ�����C��������һ����
ԭ������֮��Ϊ16�����ֶ�����Ԫ��x��y��z��Ӧ�ĵ��� X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ������µ���X��Y��Z֮����Է�����ͼ��ʾ�ı仯����֪B���������zԭ�Ӹ�����C��������һ������ش��������⣺
��1������Y�ĽṹʽΪN��N��
��2��C��X��һ�����������ɻ�����A�Ļ�ѧ����ʽ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��3�������£�C��ˮ��Һ�ܹ�ʹ��ɫʯ����ֽ�������������ӷ���ʽ��ʾ������ԭ��NH3•H2O?NH4++OH-��
��4��д��A��C��Ӧ����Y��B�Ļ�ѧ����ʽ6NO+4NH3�T5N2+6H2O��
��5���õ���ʽ��ʾB���γɹ���
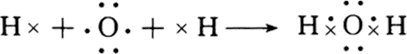 ��
��
���� ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬�������������ǵ�����������������ϡ��������⣩�����ɵ����ֻ�������NO��H2O��NH3��B���������zԭ�Ӹ�����C��������һ�����Ƚ������ַ���֪��ֻ��ˮ�����е�Hԭ�ӱȰ��������е���ԭ����һ��������ZΪHԪ�أ�A����ΪNO��B����ΪH2O��C����ΪNH3������Y��NԪ�أ�XΪOԪ�أ�������ʵĽṹ���ʽ��
��� �⣺ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬�������������ǵ�����������������ϡ��������⣩�����ɵ����ֻ�������NO��H2O��NH3��B���������zԭ�Ӹ�����C��������һ�����Ƚ������ַ���֪��ֻ��ˮ�����е�Hԭ�ӱȰ��������е���ԭ����һ��������ZΪHԪ�أ�A����ΪNO��B����ΪH2O��C����ΪNH3������Y��NԪ�أ�XΪOԪ�أ�
��1��Y��NԪ�أ���Ӧ�ĵ���Ϊ�������ṹʽΪN��N���ʴ�Ϊ��N��N��
��2��XΪ������CΪ�������ڴ��������������£�������������Ӧ����һ��������ˮ����Ӧ����ʽΪ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��3 �������£�C��NH3����ˮ��Һ�ܹ�ʹ��ɫʯ����ֽ������������ԭ���ǣ�NH3•H2O?NH4++OH-���ʴ�Ϊ��NH3•H2O?NH4++OH-��
��4��NO�Ͱ�������������ԭ��Ӧ����ˮ�͵���������ʽΪ6NO+4NH3�T5N2+6H2O���ʴ�Ϊ��6NO+4NH3�T5N2+6H2O��
��5��������ԭ�Ӻ���ԭ�ӹ��õ��ӶԶ��γɹ��ۼ������γɹ���Ϊ��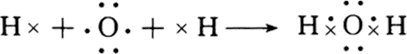 ��
��
�ʴ�Ϊ��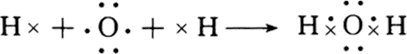 ��
��
���� ���⿼����Ԫ�ػ������ʵ��ƶϣ�Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬�����ԡ�B���������Zԭ�Ӹ�����C��������һ����Ϊ���۽����ƶϣ�������ʵĻ�ѧʽ�����������Ŀ�Ѷ��еȣ�

 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�| A�� | 9��1��6 | B�� | 3��1��2 | C�� | 3��2��2 | D�� | 9��2��6 |
 50ml0.50mol•L-1������50mL0.55mol•L-1NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȣ��ش��������⣺
50ml0.50mol•L-1������50mL0.55mol•L-1NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȣ��ش��������⣺��1����ʵ��װ���Ͽ�����ͼ��������δ�����������Ǣٻ��β�����������Ӳֽ�壨�ǰ壩����û��������װ�û��������¶ȶ���ƫС�����������С������Ӱ�족������õ��к�����ֵ��ƫС���ƫ����ƫС������Ӱ�족����
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶ȣ�t2���� | �²t2-t1���� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 28.0 | 3.0 |
| 2 | 25.1 | 25.1 | 25.1 | 28.2 | 3.1 |
| 3 | 25.1 | 25.1 | 25.1 | 28.3 | 3.2 |
��3����֪���ᡢNaOH��Һ�ܶȽ���Ϊ1.00g/cm3�кͺ���Һ�ı����� C=4.18kJ/��Kg•K���������±�����÷�Ӧ���к���Ϊ��H=-51.832kJ/mol ���к��ȣ���ϡ��Һ�У��������кͷ�Ӧ����1 molˮʱ�ķ�Ӧ�Ƚ����к��ȣ���
| A�� | ��������CCl2F2�����ƻ���������������¡�����ЧӦ�� | |
| B�� | ���������ಢ�������������������ķ�չ���� | |
| C�� | �Ͼɵ�صĻ��գ�����ҪĿ���DZ��������������ǻ��ս��� | |
| D�� | ���۲;߱���Ϊ���з�չǰ����һ���Բ;ߣ��������ڱ������� |
| A�� | 1mol�������������22.4L | |
| B�� | 1mol H2��������1g������ռ�������22.4L | |
| C�� | �ڱ�״���£�1mol�κ�������ռ�������ԼΪ22.4L•mol-1 | |
| D�� | �ڱ�״���£�1mol�κ�������ռ�������ԼΪ22.4L |
��1�����в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ�����
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| T | ������һ�ֵ���ɫ���壬�Ǻڻ�ҩ�ɷ�֮һ |
| X | �����������Ǵ�����������2�� |
| Y | �����µ���Ϊ˫ԭ�ӷ��ӣ����⻯��ˮ��Һ�ʼ��� |
| Z | Ԫ�����������+7�� |
 ����ˮ��Һ�ʼ��Ե�ԭ���ǣ��õ��뷽��ʽ��ʾ����NH3•H2O?NH4++OH-��
����ˮ��Һ�ʼ��Ե�ԭ���ǣ��õ��뷽��ʽ��ʾ����NH3•H2O?NH4++OH-����Ԫ��Z��Ԫ��T��ȣ��ǽ����Խ�ǿ����Cl����Ԫ�ط��ţ���
��̽Ѱ���ʵ����ʲ�������ѧϰ����Ҫ����֮һ��T��X��Y��Z����Ԫ�ص�����������ˮ�����л�ѧ�������Բ�ͬ���������������H2CO3���ѧʽ����
��2���������߷ֱ��ʾԪ�ص�ij��������˵�����Ĺ�ϵ��ZΪ�˵������YΪԪ�ص��й����ʣ�����������Ԫ���йص�������������߱��������Ӧ�Ŀո��У�

�٢�A��Ԫ�ص�����������������ĸ��b��
�ڵ�������Ԫ�ص�����ϼۣ�����ĸ��c��
��N3-��O2-��F-��Na+��Mg2+��Al3+�����Ӱ뾶������ĸ��a��
��3�����ڱ���ijЩԪ�ػ��������ȼ�ԡ����Ա仯��һ�������ԣ����磺
���⻯�����ȼ�ԣ��ڶ�����CH4��NH3��H2O��HF��
��������SiH4��PH3��H2S��HCl���ѧʽ����
�ڻ�����Ķ��ԣ�PH3��NH3��H2S��H2O��CS2��CO2����ѡ�������=����
| A�� | SO2��������Ư��ֽ����ë��˿����ñ�衢����ʳƷ�� | |
| B�� | ͨ�Ź��µ���Ҫ�ɷ��Ǿ���Si��̫���ܵ�صIJ�����Ҫ��SiO2 | |
| C�� | ���������Һ���ƾ���˫��ˮ����ɱ����������������ǿ������ | |
| D�� | ��������Һ����Һ���������մ������ȣ�����Һ������������� |

 ��ѧ��ѧ�г����ļ������ʴ�����ͼ��ʾ��ת����ϵ�����У�A��һ�ֺ�ɫ��ĩ״���壬C��һ�ֻ���ɫ���壮ʵ�����г���E��Һ���ն����C����ͼ�в��ֲ���ͷ�Ӧ��������ȥ����
��ѧ��ѧ�г����ļ������ʴ�����ͼ��ʾ��ת����ϵ�����У�A��һ�ֺ�ɫ��ĩ״���壬C��һ�ֻ���ɫ���壮ʵ�����г���E��Һ���ն����C����ͼ�в��ֲ���ͷ�Ӧ��������ȥ����