��Ŀ����
16��I����Ũ��Ϊ0.1mol•L-1�����ᡢ���ᡢ����������Һ���Իش���1��������Һ��c��H+������Ϊamol•L-1��bmol•L-1��cmol•L-1����С˳��Ϊb��a��c��
��2�������������������ֱ��������NaOH ��Һ��Ӧ�����ɵ��ε����ʵ�������Ϊn1mol��n2mol��n3mol�����ǵĴ�С��ϵΪn1=n2=n3��
��3���к͵���NaOH��Һ����������ʱ���ֱ�����������������������V1L��V2L��V3L�����С��ϵΪV1=2V2=V3��
II����1��̼������Һ�ʼ� �ԣ�����ᡱ�����С���������ԣ�������Һ�����������տɵõ�Na2CO3���ѧʽ�����壮
��2����֪����ͬ�������£����������ǿ��̼������ԣ�Ũ��Ϊ0.1mol•L-1CH3COONa��ҺpHΪa��Ũ��Ϊ0.1mol•L-1NaHCO3��ҺpHΪb����a��b�����������=����������
���� I����1��0.1mol•L-1�����ᡢ���ᡢ����������Һ��a=0.1mol/L��b=0.2mol/L��c��0.1mol/L��
��2������������кͷ�Ӧ���������Ũ��ʱ������ʵ�����ͬ������������غ��֪�����ε����ʵ�����ͬ��
��3���к�һ����NaOH��Һ��������ʱ��������������ʵ���Ϊ���ᡢ�����2����
II����1��Na2CO3Ϊǿ�������Σ�CO3 2-ˮ�⣺CO32-+H2O?HCO3-+OH-��ʹ��Һ�ʼ��ԣ�
��2������Խ������Ӧ�ε�ˮ��̶�Խ����Һ�ļ���Խǿ��
��� �⣺I�����ᡢ����Ϊǿ�ᣬ����Ϊ���ᣬ������ʹ���ΪһԪ�ᣬ����Ϊ��Ԫ�ᣬ
��1��0.1mol•L-1�����ᡢ���ᡢ����������Һ��a=0.1mol/L��b=0.2mol/L��c��0.1mol/L����b��a��c��
�ʴ�Ϊ��b��a��c��
��2������������кͷ�Ӧ���������Ũ��ʱ������ʵ�����ͬ������������غ��֪�����ε����ʵ�����ͬ�����ε����ʵ���Ϊn1=n2=n3��
�ʴ�Ϊ��n1=n2=n3��
��3���к�һ����NaOH��Һ��������ʱ����NaOH�����ʵ���Ϊ0.1mol���������������NaOH��ͬ��Ϊ1L��������������Ũ��������ĵ�����������٣��������Ϊ0.5L����֪����������������V1L��V2L��V3L�Ĵ�С��ϵΪV1=2V2=V3��
�ʴ�Ϊ��V1=2V2=V3��
II����1��Na2CO3Ϊǿ�������Σ�CO3 2-����ˮ�⣺CO32-+H2O?HCO3-+OH-��ʹ��Һ�ʼ��ԣ�������Һ�����������տɵõ�̼���ƹ��壻
�ʴ�Ϊ���Na2CO3��
��2����֪����ͬ�������£����������ǿ��̼������ԣ�Ũ��Ϊ0.1mol•L-1CH3COONa��ҺpHΪa��Ũ��Ϊ0.1mol•L-1NaHCO3��ҺpHΪb������Խ������Ӧ�ε�ˮ��̶�Խ����Һ�ļ���Խǿ������NaHCO3��Һ�ļ���ǿ��pH��a��b��
�ʴ�Ϊ������
���� ���⿼����ۺϣ��漰��ĵ��롢�ε�ˮ�⡢����ϵļ���ȣ����������ӵ����ʵ����Ƚϡ�����ˮ��Ӱ�����ؼ��кͷ�Ӧ��ʵ��Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��ϩ | B�� | �� | C�� | �ұ� | D�� | ���� |
| A�� | ������Ľṹ��ʽ��C5H12 | B�� | ����ı���ģ�ͣ� | ||
| C�� | ���Ȼ�̼�ĵ���ʽ�� | D�� | ��ϩ�Ľṹʽ�� |
| A�� | TI3+��Fe3+��Ag+ | B�� | Fe3+��Ag+��TI3+ | C�� | Tl+��Ag+��Fe2+ | D�� | TI3+��Ag+��Fe2+ |
| A�� | �����¶ȣ�����Ӧ���ʼ������淴Ӧ���ʼӿ죬��ѧƽ�����淴Ӧ�����ƶ� | |
| B�� | ����ѹǿ������Ӧ���淴Ӧ���ʾ�����ƽ�ⲻ�ƶ� | |
| C�� | ����A2��g����Ũ�ȣ���ѧƽ��������Ӧ�����ƶ���A2��ת�������� | |
| D�� | ����B�����ʵ�������ѧƽ��������Ӧ�����ƶ���A2��ת�������� |

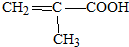 ��
��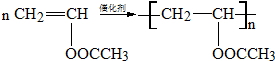 ��
��
 D���Է�������ת����
D���Է�������ת���� ϵ��
ϵ��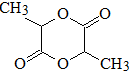 ����д��N������ȥ��Ӧ�Ļ�ѧ����ʽCH3CH��OH��COOH$��_{��}^{Ũ����}$CH2=CHCOOH+H2O��
����д��N������ȥ��Ӧ�Ļ�ѧ����ʽCH3CH��OH��COOH$��_{��}^{Ũ����}$CH2=CHCOOH+H2O�� �Ȼ���ͭ��CuCl��������ˮ���������Ҵ��İ�ɫ��ĩ•������Ũ���������HCuC12��������������ʵ���ҿ��÷�ͭм��Ũ���ᡢʳ�μ�������ȡCuCl����֪KMnO4����ϡ���ᷴӦ���ش��������⣺
�Ȼ���ͭ��CuCl��������ˮ���������Ҵ��İ�ɫ��ĩ•������Ũ���������HCuC12��������������ʵ���ҿ��÷�ͭм��Ũ���ᡢʳ�μ�������ȡCuCl����֪KMnO4����ϡ���ᷴӦ���ش��������⣺