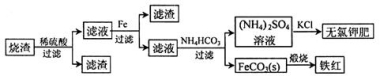
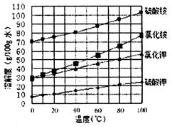
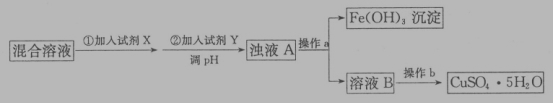
��Ŀ����
ijС��ͬѧ��̼��Ϊ�缫���CuCl2��Һʱ����������̼���ϳ����к�ɫ���������⣬����������ɫ����������Ϊ̽������̼���ϵIJ��ͬѧ���Ķ����ϲ���������¹��̣�
���й����ϣ�ͭ�Ļ�������ɫ��������

��̽��ʵ�飺
��1���������
�ٺ�ɫ����һ����ͭ����������Cu2O��
�ڰ�ɫ����Ϊͭ�Ļ�����仯ѧʽ����Ϊ��
��2��ʵ����֤
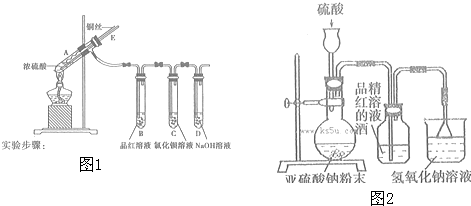
ȡ���CuCl2��Һ�������̼����ϴ �ӡ������������װ�ý���ʵ�飬��֤�������
�ӡ������������װ�ý���ʵ�飬��֤�������


��ʵ��ǰ�����װ��A�����Եķ����ǡ�
��ʵ�� ʱ����װ�ô������ҵ�����˳��ΪA������B������
ʱ����װ�ô������ҵ�����˳��ΪA������B������
��3���۲����ó�����
ʵ�������̼���ϵİ�ɫ���ʱ�Ϊ��ɫ��F�����ʲ���ɫ��D�г��ְ�ɫ���������������̼���ϵĺ�ɫ�����Ƿ���Cu2O����ǡ����������ǣ���װ�ã�����ͼ��װ�ñ�ţ��е�����˵�����������еİ�ɫ����һ�����ڣ���д��װ��b�з�����Ӧ�Ļ�ѧ����ʽ��
��ϰ��ϵ�д�
�����Ŀ
20������ʵ�顢������ؽ��۾���ȷ���ǣ�������
| A | B | C | D | |
| ʵ�� |  |  |  |  |
| ���� | Ʒ����ɫ | ����Թ���dz��ɫ���� | ���һ����Һʹ��̪����ɫ��Ϊ�ۺ�ɫ����30�벻��ԭ | ���ְ�ɫ���� |
| ���� | SO2��ǿ������ | �л����к�����ԭ�� | �ζ��ﵽ�յ� | Ksp��AgCl����Ksp��AgI�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
1�������춡����a�����㶹�أ�b��������;�㷺�����ϣ������й�˵����ȷ���ǣ�������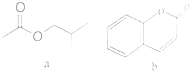

| A�� | a���ڲ������� | |
| B�� | a��b���еĹ�����������ͬ | |
| C�� | 1molb����ܺ�4molH2��Ӧ | |
| D�� | �����ʵ�����a��b�������NaOH������ͬ |
8���ܷ�����ȥ��Ӧ�����л����������ֵ��ǣ�������
| A�� | CH3-Cl | B�� | CH3CHBrCH3 | C�� | CH3CH2CHBrCH3 | D�� |  |
5��������Ԫ��X��Y��Z��ԭ���������ε���������Ԫ�ص�����������֮�͵���Z��ԭ��������Yԭ�ӵ�������������Xԭ���ڲ��������3��������Zԭ��������������3��������˵����ȷ���ǣ�������
| A�� | X��YԪ���������γɷ���ʽΪH2XY2��H4X2Y2������ | |
| B�� | ������ZY2��ֻ�������Ӽ� | |
| C�� | ��ҵ�Ͽ���X�����û�ZY�õ�Z���� | |
| D�� | Y���⻯��ķе������һ���ڵ�ͬ��Ԫ�ص��⻯��ķе�� |