��Ŀ����
(1)��ͬ���ʵ�����O2��O3������������Ŀ֮��Ϊ_______,������ԭ�ӵ����ʵ���֮��Ϊ_________��
(2)�ڱ�״���£���4 g H2����11.2 L O2����1 mol H2O�У���ԭ����������_ ___�������С����_______��������ţ�
(3)����£���224L��HCl��������835ml����=1g/cm3����ˮ�У�����������ܶ�Ϊ1.2g/cm3�� ����������ʵ���Ũ��___________.��
(4)ij������������Ϊ6.4 g������6.02��1022�����ӣ�����������Է�������Ϊ_______��
(5)Na2CO3��Ħ��������____________��0.5mol Na2CO3��������____________������________mol Na+��Na+�ĸ���ԼΪ___________��
(1) 1:1 2:3 (2) �٣� �� (3) 10 mol•L‾1
(4) 64 (5) 106g/mol�� 53g��1mol�� 6.02��1023��(ÿ��1�֣���λ��д������)
��������
�����������1��O2��O3�����ʵ�����ͬ����O2��O3�ķ�������ȣ����Է�����Ŀ֮��Ϊ1:1��O2�����к�2��Oԭ�ӣ�O3�����к�3��Oԭ�ӣ���������ԭ�ӵ����ʵ���֮��Ϊ2:3��
��2��4g H2Ϊ4g��2g/mol=2mol��Hԭ��Ϊ4mol����״����11.2L O2Ϊ11.2L��22.4L/mol=0.5mol��Oԭ��Ϊ1mol��1mol H2O��3molԭ�ӣ�����ԭ�������Ģ�4 g H2��H2O�ڱ�״���²������壬���������С����1 mol H2O��
��3���������ʵ���Ũ�ȶ���ʽ���м��㣬c(HCl)=224L��22.4L/mol��[(224L��22.4L/mol��36.5g/mol+ 835ml��1g/cm³)��1200g/L]=10mol•L‾1��
��4��6.02��1022���������ʵ���Ϊ��6.02��1022��6.02��1023mol-1=0.1mol��Ħ������Ϊ6.4g��0.1mol=64g/mol������������Է�������Ϊ64��
��5���������ԭ�����������Na2CO3��Ħ������Ϊ106g/mol����0.5mol Na2CO3������Ϊ��0.5mol��106g/mol=53g������1mol Na+��Na+�ĸ���ԼΪ6.02��1023��
���㣺���⿼���й����ʵ����ļ��㡣

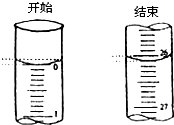 ijѧ��������֪���ʵ���Ũ�ȵ��������ζ��ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ζ��ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�