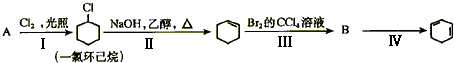
��Ŀ����
��ѧ�������ڶ�����̼�ġ����ת�����������о����ѹ��������̼ת��Ϊ��������������ʣ�
��1�������CO2��H2��1��4�ı�����ϣ�ͨ�뷴Ӧ�������ʵ��������·�Ӧ���ɻ��һ����Ҫ����Դ����������»�ѧ����ʽ��CO2+4H2��
��2������CO2��H2��1��3�ı�����ϣ�ʹ֮������Ӧ����ij����Ҫ�Ļ���ԭ�Ϻ�ˮ����ԭ�Ͽ�����
A������ B��ϩ�� C��Ȳ�� D��������
��3����֪��443��473Kʱ�����ܣ�Co��������������C5��C8�������������˹��ϳ����͵ķ���֮һ��Ҫ�ﵽ�����͵�Ҫ��CO2��H2������ȵ�ȡֵ��Χ��
��4���ס��ҡ����������ʳ������������õ�ԭ�ϲ�ͬ��������������ͬ��ԭ��
��CO(NH2)2
�����ط����еĹ�������
�ڼ׳��Խ�̿��ˮΪԭ�ϣ��ҳ�����Ȼ����ˮΪԭ�ϣ���������ϩ��ˮΪԭ�ϣ�����ҵ�йع涨������ԭ�����Ƶõ�H2��CO2�����ʵ���֮������ӽ��ϳ����ص�ԭ����NH3�������H2�����ʵ�������CO2�����ʵ���֮�ȣ����ԭ�ϵ���������ߣ��ݴ˿��жϼס��ҡ�����������ԭ����������ߵ���
��1�������CO2��H2��1��4�ı�����ϣ�ͨ�뷴Ӧ�������ʵ��������·�Ӧ���ɻ��һ����Ҫ����Դ����������»�ѧ����ʽ��CO2+4H2��
CH4
CH4
+2H2O������CO2��H2�����˹��ϳ�CnH2n+2��������д���û�ѧ����ʽ����ƽ��nCO2+��3n+1��H2��CnH2n+2+2nH2O
nCO2+��3n+1��H2��CnH2n+2+2nH2O
��2������CO2��H2��1��3�ı�����ϣ�ʹ֮������Ӧ����ij����Ҫ�Ļ���ԭ�Ϻ�ˮ����ԭ�Ͽ�����
B
B
A������ B��ϩ�� C��Ȳ�� D��������
��3����֪��443��473Kʱ�����ܣ�Co��������������C5��C8�������������˹��ϳ����͵ķ���֮һ��Ҫ�ﵽ�����͵�Ҫ��CO2��H2������ȵ�ȡֵ��Χ��
5��16��V��CO2����V��H2����8��25
5��16��V��CO2����V��H2����8��25
��4���ס��ҡ����������ʳ������������õ�ԭ�ϲ�ͬ��������������ͬ��ԭ��
|
�����ط����еĹ�������
-CO-��-NH2
-CO-��-NH2
���ýṹ��ʽ��ʾ���ڼ׳��Խ�̿��ˮΪԭ�ϣ��ҳ�����Ȼ����ˮΪԭ�ϣ���������ϩ��ˮΪԭ�ϣ�����ҵ�йع涨������ԭ�����Ƶõ�H2��CO2�����ʵ���֮������ӽ��ϳ����ص�ԭ����NH3�������H2�����ʵ�������CO2�����ʵ���֮�ȣ����ԭ�ϵ���������ߣ��ݴ˿��жϼס��ҡ�����������ԭ����������ߵ���
��
��
����������1�����������غ㶨�ɵ��۽��ͣ���ѧ��Ӧǰ��ԭ�ӵ��������Ŀ���䣮��֪�ڻ�ѧ��Ӧ����ʽ�У���Ӧ�����������������ԭ�ӵ��������Ŀ��ͬ���ɴ˿��ƶϻ�ѧ��Ӧ����ʽ�з�Ӧ���������Ļ�ѧʽ��
����Ϣ��֪��CO2��H2�����˹��ϳ�CnH2n+2��������ͬʱ����ˮ���ɣ���ƽ�γɷ���ʽ��
��2����ѧ�仯�У��仯ǰ��ԭ�����ࡢ�������䣻����CO2��H2������巢����Ӧ�ķ�������1��3������ˮ���������ӹ��ɣ����ѡ�������ݵĹ��ڻ���ԭ����C��HԪ����ɵ���Ϣ���ƶ����ɵĻ���ԭ�ϵķ�����C��Hԭ�ӵĸ����ȣ�
��3���ﵽ�����͵�Ҫ��CO2��H2������ȵ�ȡֵ��Χ�ǽ�������C5��C8������֮�䣮
��4���������صĽṹ�жϹ����ţ�
��ԭ�����Ƶõ�H2��CO2�����ʵ���֮������ӽ��ϳ����ص�ԭ����NH3�������H2�����ʵ�������CO2�����ʵ���֮�ȣ�ԭ�ϵ���������ߣ�
����Ϣ��֪��CO2��H2�����˹��ϳ�CnH2n+2��������ͬʱ����ˮ���ɣ���ƽ�γɷ���ʽ��
��2����ѧ�仯�У��仯ǰ��ԭ�����ࡢ�������䣻����CO2��H2������巢����Ӧ�ķ�������1��3������ˮ���������ӹ��ɣ����ѡ�������ݵĹ��ڻ���ԭ����C��HԪ����ɵ���Ϣ���ƶ����ɵĻ���ԭ�ϵķ�����C��Hԭ�ӵĸ����ȣ�
��3���ﵽ�����͵�Ҫ��CO2��H2������ȵ�ȡֵ��Χ�ǽ�������C5��C8������֮�䣮
��4���������صĽṹ�жϹ����ţ�
��ԭ�����Ƶõ�H2��CO2�����ʵ���֮������ӽ��ϳ����ص�ԭ����NH3�������H2�����ʵ�������CO2�����ʵ���֮�ȣ�ԭ�ϵ���������ߣ�
����⣺��1�����������غ㶨�ɵ��۽��Ϳ���֪������Ӧǰ���ԭ����Ŀ������Ӧ����ȣ���������Ļ�ѧ��Ӧ����ʽ����֪������Ӧǰ������2��O��1��C��8��H����Ӧ�������2��O��4��H��������X�к���4��H��1��C��
CO2��H2�����˹��ϳ�CnH2n+2����������Ӧ����ʽΪnCO2+��3n+1��H2��CnH2n+2+2nH2O��
�ʴ�Ϊ��CH4�� nCO2+��3n+1��H2��CnH2n+2+2nH2O��
��2���ɷ�Ӧ��CO2��H2�����һ����������1��3���������ȣ�����������Ӧ�����жϻ��������C��Hԭ�Ӹ�����Ϊ1��6�������ڷ�Ӧ������ˮ������Hԭ����Oԭ�ӻ��ϳ�ˮ���ӣ������ĸ�ѡ���е������ж�����OԪ�أ����ж�1��CO2�����е�2��Oԭ��Ӧ��4��Hԭ�ӽ�ϳ�2��ˮ���ӣ�����������е�1��Cԭ��Ӧ��2��Hԭ�ӻ��ϳɻ���ԭ�ϣ�ͨ�����Ϸ������ɵó�����ԭ����C��Hԭ�Ӹ�����ӦΪ1��2������CH4��C��Hԭ�Ӹ�����Ϊ1��4����C2H2��C��Hԭ�Ӹ�����Ϊ1��1����C2H4��C��Hԭ�Ӹ�����Ϊ1��2����C2H6��C��Hԭ�Ӹ�����Ϊ1��3��
�ʴ�Ϊ��B��
��3���� ��1����֪nCO2+��3n+1��H2��CnH2n+2+2nH2O��������C5H12ʱ��n����3n+1��=5��16��
������C8H18ʱ��n����3n+1��=8��25��
�ʴ�Ϊ��5��16��V��CO2����V��H2����8��25
��4�������ط��ӵĽṹΪ �����Է����еĹ�������-CO-��-NH2��
�����Է����еĹ�������-CO-��-NH2��
�ʴ�Ϊ��-CO-��-NH2
��NH3�������H2�����ʵ�������CO2�����ʵ���֮��Ϊ2��1�ϳ����أ���H2��CO2�����ʵ���֮��Ϊ
��1=3��1��
�׳��Խ�̿��ˮΪԭ�ϣ�C+2H2O
CO2+2H2�����ɵ�H2��CO2�����ʵ���֮��Ϊ2��1��
�ҳ�����Ȼ����ˮΪԭ�ϣ�CH4+2H2O
CO2+4H2�����ɵ�H2��CO2�����ʵ���֮��Ϊ2��1��
��������ϩ��ˮΪԭ�ϣ�C2H4+4H2O
2CO2+6H2�����ɵ�H2��CO2�����ʵ���֮��Ϊ3��1��
�ʴ�Ϊ����
CO2��H2�����˹��ϳ�CnH2n+2����������Ӧ����ʽΪnCO2+��3n+1��H2��CnH2n+2+2nH2O��
�ʴ�Ϊ��CH4�� nCO2+��3n+1��H2��CnH2n+2+2nH2O��
��2���ɷ�Ӧ��CO2��H2�����һ����������1��3���������ȣ�����������Ӧ�����жϻ��������C��Hԭ�Ӹ�����Ϊ1��6�������ڷ�Ӧ������ˮ������Hԭ����Oԭ�ӻ��ϳ�ˮ���ӣ������ĸ�ѡ���е������ж�����OԪ�أ����ж�1��CO2�����е�2��Oԭ��Ӧ��4��Hԭ�ӽ�ϳ�2��ˮ���ӣ�����������е�1��Cԭ��Ӧ��2��Hԭ�ӻ��ϳɻ���ԭ�ϣ�ͨ�����Ϸ������ɵó�����ԭ����C��Hԭ�Ӹ�����ӦΪ1��2������CH4��C��Hԭ�Ӹ�����Ϊ1��4����C2H2��C��Hԭ�Ӹ�����Ϊ1��1����C2H4��C��Hԭ�Ӹ�����Ϊ1��2����C2H6��C��Hԭ�Ӹ�����Ϊ1��3��
�ʴ�Ϊ��B��
��3���� ��1����֪nCO2+��3n+1��H2��CnH2n+2+2nH2O��������C5H12ʱ��n����3n+1��=5��16��
������C8H18ʱ��n����3n+1��=8��25��
�ʴ�Ϊ��5��16��V��CO2����V��H2����8��25
��4�������ط��ӵĽṹΪ
 �����Է����еĹ�������-CO-��-NH2��
�����Է����еĹ�������-CO-��-NH2���ʴ�Ϊ��-CO-��-NH2
��NH3�������H2�����ʵ�������CO2�����ʵ���֮��Ϊ2��1�ϳ����أ���H2��CO2�����ʵ���֮��Ϊ
| 2��3 |
| 2 |
�׳��Խ�̿��ˮΪԭ�ϣ�C+2H2O
| ||
�ҳ�����Ȼ����ˮΪԭ�ϣ�CH4+2H2O
| ||
��������ϩ��ˮΪԭ�ϣ�C2H4+4H2O
| ||
�ʴ�Ϊ����
���������⿼��ѧ�����û�ѧ��Ӧ����ʽ�������غ㶨�����ƶ����ʵĻ�ѧʽ���غ㷽���ǽ����ij��÷�����ѧ��Ӧ��ϤԪ���غ㡢ԭ���غ���������ϰ�⣮

��ϰ��ϵ�д�
�����Ŀ
 ����������һ�������
����������һ�������


 ����������һ�������
����������һ�������