��Ŀ����
A��B��C��D��E��F���ֻ��������A��B��C��D��E���ɶ�����Ԫ����ɣ���ɫ��Ӧ���ʻ�ɫ��B��C��E��������Ԫ����ɣ�B��C�����Ԫ����ͬ����C��Ħ��������B��80g/mol��ش��������⣺
��1�����廯����AΪdz��ɫ��ĩ���û������к��еĻ�ѧ��Ϊ ������ţ���
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
��2����ΪB��Fʵ��IJ������ݣ�
д��B��ϡH2S04��Ӧ�����ӷ���ʽ�� ��д�����з�Ӧ�Ļ�ѧ����ʽ�� ��
��3������6������Mn2+��MnO4-��H+��H20��X2Y82-��C�к��е������ӣ���XY42-���һ�����ӷ���ʽ����֪Mn2+Ϊ��ԭ�����õ�1mol MnO4-�������������ʵ���Ϊ ��
��4��������D��E�����ת����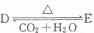 ������D��E?XH20�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ ��E?XH20�Ļ�ѧʽΪ ��
������D��E?XH20�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ ��E?XH20�Ļ�ѧʽΪ ��
��1�����廯����AΪdz��ɫ��ĩ���û������к��еĻ�ѧ��Ϊ
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D�����
��2����ΪB��Fʵ��IJ������ݣ�
| ��� | ��Ҫʵ�鲽�輰ʵ������ |
| �� | �ں���B����Һ�У�����ϡH2S04������dz��ɫ���Ǻ���ɫ�д̼�����ζ�����壮 |
| �� | 20 ml��ˮ�еμ�F�ı�����Һ1��2ml������Һ��ʺ��ɫ |
| �� | ��ʵ��ڵõ��ĺ��ɫҺ��������������գ����յõ�����ɫ���� |
��3������6������Mn2+��MnO4-��H+��H20��X2Y82-��C�к��е������ӣ���XY42-���һ�����ӷ���ʽ����֪Mn2+Ϊ��ԭ�����õ�1mol MnO4-�������������ʵ���Ϊ
��4��������D��E�����ת����
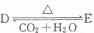 ������D��E?XH20�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ
������D��E?XH20�Ļ����13.04g�����ȵ���ȫ��Ӧ���������ͨ��ŨH2SO4����3.42g��ʣ������ͨ����ʯ������2.20g����������D������Ϊ���㣺������ƶ�
ר�⣺�ƶ���
��������1��A��B��C��D��E���ɶ�����Ԫ����ɣ���ɫ��Ӧ���ʻ�ɫ��������NaԪ�أ����廯����AΪdz��ɫ��ĩ�����ǹ������ƣ�
��2�����ݢڢۿ�֪F���Ȼ�����Һ�����ݢٿ�֪B��NaS2O3��
��3��Mn2+����������MnO4-�����ϼ�����5����λ����������X2Y82һ���仹ԭ������SO42-��1mol�������õ�2mol���ӣ����ݵ��ӵĵ�ʧ�غ������Ҫ�����������ʵģ�
��4������ת����֪D��̼�����ƣ�EΪ̼���ƣ�Ũ�������ӵ���������ˮ����������ʯ�����ӵ�������CO2������������ˮ��������̼�����ʵ��������ݶ�����̼����̼�����Ƶ����ʵ�������������E?XH2O���������������ˮ�����ʵ�������E?XH2O�нᾧˮ�����ʵ������ټ���E?XH2O��̼���Ƶ����ʵ���������ȷ����ѧʽ��
��2�����ݢڢۿ�֪F���Ȼ�����Һ�����ݢٿ�֪B��NaS2O3��
��3��Mn2+����������MnO4-�����ϼ�����5����λ����������X2Y82һ���仹ԭ������SO42-��1mol�������õ�2mol���ӣ����ݵ��ӵĵ�ʧ�غ������Ҫ�����������ʵģ�
��4������ת����֪D��̼�����ƣ�EΪ̼���ƣ�Ũ�������ӵ���������ˮ����������ʯ�����ӵ�������CO2������������ˮ��������̼�����ʵ��������ݶ�����̼����̼�����Ƶ����ʵ�������������E?XH2O���������������ˮ�����ʵ�������E?XH2O�нᾧˮ�����ʵ������ټ���E?XH2O��̼���Ƶ����ʵ���������ȷ����ѧʽ��
���
�⣺��1��A��B��C��D��E���ɶ�����Ԫ����ɣ���ɫ��Ӧ���ʻ�ɫ��������NaԪ�أ����廯����AΪdz��ɫ��ĩ�����ǹ������ƣ����������к������Ӽ��ͷǼ��Լ����ʴ�Ϊ��AC��
��2�����ݢڢۿ�֪F���Ȼ�����Һ�����ݢٿ�֪B��NaS2O3��B�����ᷴӦ�ķ���ʽΪ��S2O32-+2H+=S��+SO2��+H2O����������������������Ʊ�������ʽΪ��FeCl3+3H2O
Fe��OH��3�����壩+3HCl��
�ʴ�Ϊ��S2O32-+2H+=S��+SO2��+H2O��FeCl3+3H2O
Fe��OH��3�����壩+3HCl��
��3��Mn2+����������MnO4-�����ϼ�����5����λ����������X2Y82һ���仹ԭ������SO42-��1mol�������õ�2mol���ӣ����Ը��ݵ��ӵĵ�ʧ�غ��֪����Ҫ�����������ʵ�����
=2.5mol���ʴ�Ϊ��2.5mol��
��4������ת����֪D��̼�����ƣ�EΪ̼���ƣ�Ũ�������ӵ���������ˮ�����������ʵ�����
=0.19mol����ʯ�����ӵ�������CO2������������CO2�����ʵ�����
=0.05mol����
2NaHCO3
Na2CO3+CO2��+H2O
0.1mol 0.05mol 0.05mol
���D��NaHCO3�������ʵ�����0.1mol��������0.1mol��84g/mol=8.4g��
����Na2CO3?XH2O��������13.04g-8.4g=4.64g�����Na2CO3?XH2O�нᾧˮ�����ʵ�����0.19mol-0.05mol=0.14mol��������0.14mol��18g/mol=2.52g������Na2CO3?XH2O��Na2CO3��������4.64g-2.52g=2.12g�������ʵ�����
=0.02mol����n��Na2CO3����n��H2O��=0.02mol��0.14mol=1��7����E?XH20�Ļ�ѧʽΪNa2C03?7H2O��
�ʴ�Ϊ��8.4g��Na2C03?7H2O��
��2�����ݢڢۿ�֪F���Ȼ�����Һ�����ݢٿ�֪B��NaS2O3��B�����ᷴӦ�ķ���ʽΪ��S2O32-+2H+=S��+SO2��+H2O����������������������Ʊ�������ʽΪ��FeCl3+3H2O
| ||
�ʴ�Ϊ��S2O32-+2H+=S��+SO2��+H2O��FeCl3+3H2O
| ||
��3��Mn2+����������MnO4-�����ϼ�����5����λ����������X2Y82һ���仹ԭ������SO42-��1mol�������õ�2mol���ӣ����Ը��ݵ��ӵĵ�ʧ�غ��֪����Ҫ�����������ʵ�����
| 1mol��5 |
| 2 |
��4������ת����֪D��̼�����ƣ�EΪ̼���ƣ�Ũ�������ӵ���������ˮ�����������ʵ�����
| 3.42g |
| 18g/mol |
| 2.2g |
| 44g/mol |
2NaHCO3
| ||
0.1mol 0.05mol 0.05mol
���D��NaHCO3�������ʵ�����0.1mol��������0.1mol��84g/mol=8.4g��
����Na2CO3?XH2O��������13.04g-8.4g=4.64g�����Na2CO3?XH2O�нᾧˮ�����ʵ�����0.19mol-0.05mol=0.14mol��������0.14mol��18g/mol=2.52g������Na2CO3?XH2O��Na2CO3��������4.64g-2.52g=2.12g�������ʵ�����
| 2.12g |
| 106g/mol |
�ʴ�Ϊ��8.4g��Na2C03?7H2O��
���������⿼�������ƶϡ���ѧ��������ʽ��д��������ԭ��Ӧ����������ȣ��Ѷ��еȣ���Ҫѧ���߱���ʵ�Ļ������ǶԻ���֪ʶ���ۺ�Ӧ�ã�

��ϰ��ϵ�д�
�����Ŀ
��˿��ͭƬ��������ȼ�յ��̵���ɫ�ֱ�Ϊ��������
| A���ػơ��غ� |
| B���ػơ��� |
| C���غ졢�� |
| D���غ졢�ػ� |
��NH4Cl��NH3?H2O��ɵĻ��Һ�У����й�ϵһ����ȷ���ǣ�������
| A��c��NH4+����c��Cl-�� |
| B��c��H+��=c��OH-�� |
| C��c��NH4+��+c��NH3?H2O����c��Cl-�� |
| D��c��NH4+����c��Cl-��+c��OH-�� |
���������ӷ�Ӧ����ʽ���Ӧ�Ļ�ѧ����ʽ��ȷ���ǣ�������
| A��Zn2++2OH-=Zn��OH��2�� ZnCO3+2NaOH=Zn��OH��2��+Na2CO3 |
| B��Ba2++SO42-=BaSO4�� Ba��OH��2+H2SO4=BaSO4��+2H2O |
| C��Ag++Cl-=AgCl�� AgNO3+NaCl=AgCl��+NaNO3 |
| D��Cu+2Ag+=Cu2++2Ag�� Cu+2AgCl=2Ag+CuCl2 |
�����л������������ǣ�������
| A����ϩ | B���Ҵ� | C������ | D�������� |
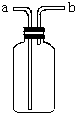 ��ͼ��ʾװ���ǻ�ѧʵ���г�������������������ϴ���⣬����������;��
��ͼ��ʾװ���ǻ�ѧʵ���г�������������������ϴ���⣬����������;��