��Ŀ����
10��NA��ʾ�����ӵ�������ֵ������˵���У���ȷ���ǣ��������ٳ����£�15g��ȩ��
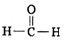 ���к��еĹ��õ��Ӷ�����Ϊ2NA
���к��еĹ��õ��Ӷ�����Ϊ2NA�ڳ��³�ѹ�£�18g D2O�к��еĵ�������Ϊ10NA
�۽�l 00mL 0.1mol•L-1��FeCl3��Һ�����ˮ�У����Ƶ�Fe��OH��3����0.0l NA��
���ڷ�ӦKIO3+6HI�TKI+3I2+3H2O�У�ÿ����3mol I2ת�Ƶĵ�����Ϊ5NA
�ݵ�⾫��ͭʱ������·��ת��NA�����ӣ������ܽ�32gͭ
��1.7g���ǻ���1.7g������������������������Ϊ0.9NA��
| A�� | �٢� | B�� | �ۢ� | C�� | �ڢ� | D�� | �ڢ� |
���� ������ת��Ϊ���ʵ��������1����ȩ���Ӻ���4�Թ��õ��ӶԽ��
��D2OĦ������Ϊ20g/mol������10�����ӣ�
��������������Ϊ������ӵļ����壻
���ڷ�ӦKIO3+6HI�TKI+3I2+3H2O�У�ת��5mol���ӣ�
�ݵ�⾫��ͭʱ���������Ϸŵ�IJ�ֹ��ͭ��
��1���ǻ�����9�����ӣ�1�����������Ӻ���10�����ӣ�
��� �⣺�ٳ����£�15g��ȩ���ʵ���Ϊ$\frac{15g}{30g/mol}$=0.5mol�����еĹ��õ��Ӷ�����Ϊ0.5mol��4��NA=2NA������ȷ��
�ڳ��³�ѹ�£�18g D2O�к��еĵ�������Ϊ$\frac{18g}{20g/mol}$��NA=9NA���ʴ���
��������������Ϊ������ӵļ����壬���Խ�l 00mL 0.1mol•L-1��FeCl3��Һ�����ˮ�У����Ƶ�Fe��OH��3����С��0.0l NA���ʴ���
���ڷ�ӦKIO3+6HI�TKI+3I2+3H2O�У�ת��5mol���ӣ�����3mol�⣬����ȷ��
�ݵ�⾫��ͭʱ���������Ϸŵ�IJ�ֹ��ͭ�����б�ͭ���õ����ʣ��ʵ���·��ת��NA������ʱ���������ܽ��ͭ������С��32g���ʴ���
��1.7g���ǻ���1.7g���������������ʵ�������1mol�������������ֱ�Ϊ0.9NA��NA���ʴ���
��ѡ��A��
���� ���⿼�����ʵ����ļ��㣬��Ŀ�ѶȲ���ע�����պ������ʵ���Ϊ���ĵĸ���ѧ���밢���ӵ������Ĺ�ϵ��ע����ȷ�ǻ������������ӵ�����

| ѡ�� | A | B | C | D |
| ʵ����� | ��MgCl2��AlCl3��Һ�У���1mol������μ���NaOH��Һ | ��HCl��MgCl2��AlCl3��NH4Cl��Һ�У���1mol������μ���NaOH��Һ | ��NaOH��NaAlO2��Һ�У���1mol������μ���HCl��Һ | ��NaOH��Na2CO3�����Һ�У���1mol���μ�ϡ���� |
| ͼ�� |  |  |  |  |
| A�� | A | B�� | B | C�� | C | D�� | D |
S��s��+2K��s��=K2S��s����H2=b kJ•mol-1
2K��s��+N2��g��+3O2��g��=2KNO3��s������H3=c kJ•mol-1
�ڻ�ҩ���й��Ŵ����Ĵ���֮һ���䱬ը���Ȼ�ѧ����ʽΪ��
S��s��+2KNO3��s��+3C��s��=K2S��s��+N2��g��+3CO2��g����H=x kJ•mol-1�� ��xΪ��������
| A�� | 3a+b-c | B�� | c+3a-b | C�� | a+b-c | D�� | c+a-b |
| A�� | pH=4��0.1mol/L NaHA��Һ��c��HA-����c��H+����c��A2-����c��OH-����c��H2A�� | |
| B�� | 10mL 0.1mol/L CH3COOH��Һ��20mL 0.1mol/L NaOH��Һ��Ϻ���Һ������Ũ�ȹ�ϵ��c��OH-��=c��H+��+c��CH3COO-��+2c��CH3COOH�� | |
| C�� | ���ִ�����Һ�����ʵ���Ũ�ȷֱ�Ϊc1��c2��pH�ֱ�Ϊa��a+1����c1��10c2 | |
| D�� | ��֪��HAΪ���ᣬ��������Һ��0.1mol/LHA��Һ����0.3mol/LHA��Һ��0.1mol/LNaOH��Һ������Ļ��Һ��c��H+���٣��� |
| A�� | ������ԭ����ͭ | |
| B�� | ������̼������������Һ��������̼������ | |
| C�� | ����ͨ���Ȼ�����Һ���������Ȼ����������� | |
| D�� | ����طֽ������� |
| A�� | ���������������������ֱ���ȫȼ�գ����߷ų��������� | |
| B�� | ��C�����ʯ����C��ʯī����H=-1.9KJ/mol ��֪�����ʯ��ʯī�ȶ� | |
| C�� | ��101Kpaʱ��2gH2��ȫȼ������Һ̬ˮ���ų�285.8KJ����������ȼ�յ��Ȼ�ѧ����ʽΪ��2H2��g��+O2��g��=2H2O��l����H=+285.8KJ/mol | |
| D�� | ϡ��Һ�У�H+��aq��+OH-��aq��=H2O��l����H=-53.7KJ/mol��������1 mol CH3COOH�뺬1 mol NaOH����Һ��ϣ��ų�������С��53.7KJ |


 �����õ����ű�ʾ�÷�Ӧ����ת�Ƶķ������Ŀ��
�����õ����ű�ʾ�÷�Ӧ����ת�Ƶķ������Ŀ�� ������һ�����ʵĵ���ʽ��
������һ�����ʵĵ���ʽ��