��Ŀ����
18��25��ʱ��������Һ���й��������ʵ���Ũ�ȹ�ϵ������ǣ�������| A�� | pH=4��0.1mol/L NaHA��Һ��c��HA-����c��H+����c��A2-����c��OH-����c��H2A�� | |
| B�� | 10mL 0.1mol/L CH3COOH��Һ��20mL 0.1mol/L NaOH��Һ��Ϻ���Һ������Ũ�ȹ�ϵ��c��OH-��=c��H+��+c��CH3COO-��+2c��CH3COOH�� | |
| C�� | ���ִ�����Һ�����ʵ���Ũ�ȷֱ�Ϊc1��c2��pH�ֱ�Ϊa��a+1����c1��10c2 | |
| D�� | ��֪��HAΪ���ᣬ��������Һ��0.1mol/LHA��Һ����0.3mol/LHA��Һ��0.1mol/LNaOH��Һ������Ļ��Һ��c��H+���٣��� |
���� A��pH=4��0.1mol/L NaHA��Һ�У�HA-���ӵ��������ˮ����Һ�����ԣ�
B.10mL 0.1mol/L CH3COOH��Һ��20mL 0.1mol/L NaOH��Һ��Ϻ�õ������ƺ�����������Һ����Һ�д��ڵ���غ�������غ���������
C������ĵ���̶Ⱥ����Ũ�ȴ�С�йأ�Ũ��Խ�����̶�ԽС��
D.0.1mol/LHA��Һ�е���ƽ����Һ�����ԣ�0.3mol/LHA��Һ��0.1mol/LNaOH��Һ������Ļ�ϵõ�HA��NaA�����Һ��A-���Ӷ�HA�������������ã�
��� �⣺A��pH=4��0.1mol/L NaHA��Һ�У�HA-���ӵ��������ˮ����Һ�����ԣ�����Ũ��c��HA-����c��H+����c��A2-����c��OH-����c��H2A������A��ȷ��
B.10mL 0.1mol/L CH3COOH��Һ��20mL 0.1mol/L NaOH��Һ��Ϻ�õ������ƺ�����������Һ����Һ�д��ڵ���غ�c��OH-��+c��CH3COO-��=c��H+��+c��Na+���������غ�Ϊ��2c��CH3COO-��+2c��CH3COOH��=c��Na+�����������õ�c��OH-��=c��H+��+c��CH3COO-��+2c��CH3COOH������B��ȷ��
C���������Ũ��ԽС�����Խ��pHԽ��c1��1=10-a��c2��2=10-a-1����1����2������c1��10 c2����C����
D.0.1mol/LHA��Һ�е���ƽ����Һ�����ԣ�0.3mol/LHA��Һ��0.1mol/LNaOH��Һ������Ļ�ϵõ�HA��NaA�����Һ����Һ��HA�Ѷ�Ϊ0.1mol/L��A-���Ӷ�HA�������������ã�������Ũ�Ȣ٣��ڣ���D��ȷ��
��ѡC��
���� ���⿼���˵������Һ������Ũ�ȴ�С�Ƚϡ�������ʵ���ƽ�⡢��Һ�е���غ�������غ������������ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | �ô����ȥˮ���е�̼��ƣ�CaCO3+2H+=Ca2++H2O+CO2�� | |
| B�� | ʯ��ˮ�м������С�մ���Һ��HCO3-+Ca2++OH-=CaCO3��+H2O | |
| C�� | �ⱥ��NaCl������Һ��2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2OH-+H2��+Cl2�� | |
| D�� | ����������������Һ��Al+2OH-+H2O=AlO2-+2H2�� |
| A�� | ���ʵ����������о������ӵ�һ�����ʻ������������䵥λ��Ħ�� | |
| B�� | �����Ħ��������¶Ⱥ�ѹǿ�йأ�ѹǿԽ�����Խ�� | |
| C�� | ��x��N ������ԭ�ӵ��ʼ���1�ˣ����ӵ������ɱ�ʾΪ14x/mol | |
| D�� | �κ�һ�������ӣ���Ħ��������g/molΪ��λʱ������ֵ���������ӵ���Է������������ԭ��������ͬ |
| A�� | 12 g12C�����е�̼ԭ����ΪNA�� | |
| B�� | NA�Ľ���ֵΪ6.02��1023 | |
| C�� | 2 mol H2O���е�H2O������ĿΪ2NA�� | |
| D�� | 2NA��Cl2������Ϊ71 g |
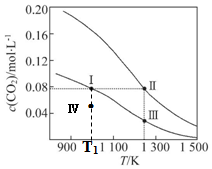
| A�� | �÷�Ӧ�ġ�H��0����S��0 | B�� | ��ϵ����ѹǿp��p ����p ���� | ||
| C�� | ƽ�ⳣ����K����K ���� | D�� | T1Kʱ������������״̬�� v��������v���棩 |
| A�� | ���������Ũ�ȵ�K2CO3��Һ�������ϣ�����Һ�У�c��K+����c��Cl-����c��HCO3-����c��OH-����c��H+�� | |
| B�� | ��ˮ�еμ����������ԣ�����Һ�У�c��NH4+��=c��Cl-����c��OH-��=c��H+�� | |
| C�� | ���������Ũ�ȵ�CH3COOH��Һ��NaOH��Һ��Ϻ���Һ��pH=8����c��OH-��-c��CH3COOH��=1��10-8mol•L-1 | |
| D�� | ���������Ũ�Ȣ�NaCl����CH3COONa����NaClO��Һ������������С˳�ۣ��ڣ��� |
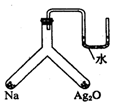 ��ּ�����ͼ��ʾ���ܱ������з������Լ�������λ�ã�����������������Ӧ���һָ���ԭ�����¶�ʱ��U�ι���������Һ����ƽ�������й�˵��������ǣ�������
��ּ�����ͼ��ʾ���ܱ������з������Լ�������λ�ã�����������������Ӧ���һָ���ԭ�����¶�ʱ��U�ι���������Һ����ƽ�������й�˵��������ǣ�������| A�� | װ���ڿ����ɷֱ��ֲ��� | B�� | ���ȶ��ԣ��Ƶ�������ǿ��Ag2O | ||
| C�� | װ��������Ag2O���ʵ�����Ϊ2��1 | D�� | �е���ɫ�������� |
| A�� | ��pH=5��HCl ��Һϡ��1000����pH��Ϊ8 | |
| B�� | ��pH=8��NaOH��Һϡ��1000����pH��Ϊ6 | |
| C�� | �� pH=2��HCl ��Һ���������������Ϊԭ����$\frac{1}{10}$��pH��Ϊ1 | |
| D�� | ��pH=3�Ĵ�����Һϡ��100����pH��5 |
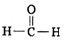 ���к��еĹ��õ��Ӷ�����Ϊ2NA
���к��еĹ��õ��Ӷ�����Ϊ2NA