��Ŀ����
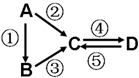 A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʣ�����֮�������µķ�Ӧ��ϵ��
A��B��C��D��Ϊ��ѧ��ѧ�����Ĵ����A�ǵ��ʣ�����֮�������µķ�Ӧ��ϵ����1����A�ǵ���ɫ���壬C��D���������C������������Ҫ���ʣ�����C���ʿɵõ��м�ֵ�Ļ�ѧƷ��д���û�ѧƷ�е�1�����1���ε�����
��2����B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ��B��C��һ�������·�Ӧ���ɵ�A�Ǵ�����Ҫ�ɷ֣�д���÷�Ӧ�Ļ�ѧ����ʽ
��3����D���ʾ������ԣ��ڢ۷�Ӧ��Ҫ��ǿ����Һ���ܷ�Ӧ��ͨ�������һ����������ЧӦ����Ҫ���壮�жϵ���A��Ԫ�������ڱ��е�λ����
��4����A��Ӧ����㷺�Ľ������ܷ�Ӧ�õ�A���ڢݷ�Ӧ���õ�ͬһ�ַǽ������ʣ�C����Һ����ʴ��ӡˢͭ��·�壬д�÷�Ӧ�����ӷ���ʽ
���㣺������ƶ�
ר�⣺�ƶ���
��������1��C������������Ҫ������C��SO2��A�ǵ���ɫ���壬��A��S��D��SO3���û�ѧƷ�е�һ���������ᣬһ����������泥��ƣ���
��2��B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ����C��NO��D��NO2��B��NH3��A��N2��
��3��D���ʾ������ԣ��ڢ۷�Ӧ��Ҫ��ǿ����Һ���ܷ�Ӧ��ͨ�������һ����������ЧӦ����Ҫ���壬��D��Al��OH��3��C��NaAlO2����A��Al��B��Al2O3��
��4��A��Ӧ����㷺�Ľ������ܷ�Ӧ�õ�A����AΪFe��C����Һ����ʴ��ӡˢͭ��·�壬C��FeCl3���ڢݷ�Ӧ�õ��ķǽ���ΪCl2������D��FeCl2���Դ������
��2��B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ����C��NO��D��NO2��B��NH3��A��N2��
��3��D���ʾ������ԣ��ڢ۷�Ӧ��Ҫ��ǿ����Һ���ܷ�Ӧ��ͨ�������һ����������ЧӦ����Ҫ���壬��D��Al��OH��3��C��NaAlO2����A��Al��B��Al2O3��
��4��A��Ӧ����㷺�Ľ������ܷ�Ӧ�õ�A����AΪFe��C����Һ����ʴ��ӡˢͭ��·�壬C��FeCl3���ڢݷ�Ӧ�õ��ķǽ���ΪCl2������D��FeCl2���Դ������
���
�⣺��1��C������������Ҫ������C��SO2��A�ǵ���ɫ���壬��A��S��D��SO3���û�ѧƷ�е�һ���������ᣬ���ü���ᣬһ����������泥��ƣ������û�ѧƷ�е�1�����1���ε����Ʒֱ�Ϊ���ᡢ����泥��ƣ����ʴ�Ϊ���������泥��ƣ���
��2��B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ����C��NO��D��NO2��B��NH3��A��N2��B��C�ķ�ӦΪ4NH3+6NO
5N2+6H2O��
�ʴ�Ϊ��4NH3+6NO
5N2+6H2O��
��3��D���ʾ������ԣ��ڢ۷�Ӧ��Ҫ��ǿ����Һ���ܷ�Ӧ��ͨ�������һ����������ЧӦ����Ҫ���壬��D��Al��OH��3��C��NaAlO2����A��Al��B��Al2O3��Al�����ڱ��е�λ���ǵ������ڢ�A�壬�ܷ�Ӧ���ӷ���ʽΪAlO2-+2H2O+CO2=Al��OH��3��+HCO3-��
�ʴ�Ϊ���������ڢ�A�壻AlO2-+2H2O+CO2=Al��OH��3��+HCO3-��
��4��A��Ӧ����㷺�Ľ������ܷ�Ӧ�õ�A����AΪFe��C����Һ����ʴ��ӡˢͭ��·�壬C��FeCl3���ڢݷ�Ӧ�õ��ķǽ���ΪCl2������D��FeCl2����C����Һ����ʴ��ӡˢͭ��·������ӷ���ʽΪCu+2Fe3+=Cu2++2Fe2+���ʴ�Ϊ��Cu+2Fe3+=Cu2++2Fe2+��
��2��B����̬�⻯�C��D���������һ���ɹ⻯ѧ������Ⱦ����C��NO��D��NO2��B��NH3��A��N2��B��C�ķ�ӦΪ4NH3+6NO
| ||
�ʴ�Ϊ��4NH3+6NO
| ||
��3��D���ʾ������ԣ��ڢ۷�Ӧ��Ҫ��ǿ����Һ���ܷ�Ӧ��ͨ�������һ����������ЧӦ����Ҫ���壬��D��Al��OH��3��C��NaAlO2����A��Al��B��Al2O3��Al�����ڱ��е�λ���ǵ������ڢ�A�壬�ܷ�Ӧ���ӷ���ʽΪAlO2-+2H2O+CO2=Al��OH��3��+HCO3-��
�ʴ�Ϊ���������ڢ�A�壻AlO2-+2H2O+CO2=Al��OH��3��+HCO3-��
��4��A��Ӧ����㷺�Ľ������ܷ�Ӧ�õ�A����AΪFe��C����Һ����ʴ��ӡˢͭ��·�壬C��FeCl3���ڢݷ�Ӧ�õ��ķǽ���ΪCl2������D��FeCl2����C����Һ����ʴ��ӡˢͭ��·������ӷ���ʽΪCu+2Fe3+=Cu2++2Fe2+���ʴ�Ϊ��Cu+2Fe3+=Cu2++2Fe2+��
���������⿼��������ƶϣ�Ϊ��Ƶ���㣬���ճ������ʵ����ʡ�����֮���ת����ӦΪ���Ĺؼ������ط������ƶ��������ۺϿ��飬ע�ⳣ�����������뻷����Ⱦ���⣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
ijԪ�صĵ����ܣ����ӷ��أ����£�
��Ԫ��λ��Ԫ�����ڱ��������ǣ�������
| I1 | I2 | I3 | I4 | I5 | I6 | I7 |
| 14.5 | 29.6 | 47.4 | 77.5 | 97.9 | 551.9 | 666.8 |
| A��IA | B����A | C����A | D����A |
���м���Ԫ�صı���ʽ������ǣ�������
A��F-�ĵ����Ų�ͼ��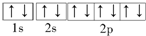 |
B��Na+�Ľṹʾ��ͼ��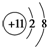 |
| C��Mg2+�ĵ����Ų�ʽ��1s22s22p6 |
| D��Cr�ļ����Ų�ʽ��[Ar]3d44s2 |
����ԭ�Ӹ����Ӳ��е��������������ǣ�������
| A��Sc��K��2��L��8��M��8��N��3�� |
| B��Cr��K��2��L��8��M��13��N��1�� |
| C��Ge��K��2��L��8��M��18��N��4�� |
| D��Cu��K��2��L��8��M��18��N��1�� |
 25��ʱ����25mL0.1mol/L������������Һ�У���μ���0.2mol/LCH3COOH����Һ��pH�ı仯������ͼ��ʾ�����з����Ľ�����ȷ���ǣ�������
25��ʱ����25mL0.1mol/L������������Һ�У���μ���0.2mol/LCH3COOH����Һ��pH�ı仯������ͼ��ʾ�����з����Ľ�����ȷ���ǣ�������| A����B��ĺ�����a=12.5������c��Na+��=c��CH3COO-�� |
| B����������A��B���κ�һ�㣬��Һ�ж��У�c��Na+����c��OH-����c��CH3COO-����c��H+�� |
| C��D��ʱ��c��CH3COO-��+c��CH3COOH��=c��Na+�� |
| D��C��ʱ��c��CH3COO-��=c��Na+����c��H+��=c��OH-�� |
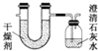 ��֪ij��ȼ�Ϻ���̼�������Ԫ�أ�Ϊ�˲ⶨ����ȼ����̼��������Ԫ�ص������ȣ��ɽ���̬ȼ�Ϸ���������O2��ȼ�գ���������������ȫ��ͨ��ͼʾװ�ã��õ�������е�ʵ�����ݣ�������������ȫ�����գ���
��֪ij��ȼ�Ϻ���̼�������Ԫ�أ�Ϊ�˲ⶨ����ȼ����̼��������Ԫ�ص������ȣ��ɽ���̬ȼ�Ϸ���������O2��ȼ�գ���������������ȫ��ͨ��ͼʾװ�ã��õ�������е�ʵ�����ݣ�������������ȫ�����գ��� ���Ļ�����������������������Ҫ�ĵ�λ����ش��������⣺
���Ļ�����������������������Ҫ�ĵ�λ����ش��������⣺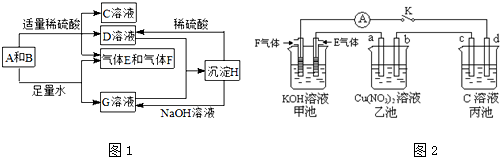
 ����������Ļ�ѧʽΪ
����������Ļ�ѧʽΪ