��Ŀ����
ʵ��������500mL 0.1mol?L-1������������Һ��ʵ�鲽�����£�
a��������Ҫ�������ƹ����������
b�������������ƹ��壮
c�����ձ��е���Һע������ƿ��������������ˮϴ���ձ��ڱ�2��3�Σ�ϴ��ҺҲע������ƿ��
d��������������ˮ�ܽ�����õ��������ƹ��壬��ȴ��
e���Ǻ�ƿ�����������µߵ���ҡ�ȣ�
f������������ƿ�м�����ˮ���̶�����1��2cmʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
�ش��������⣺
��1������� g���������ƣ��������ƹ���Ӧ���� �г�����
��2�������������ȷ����˳���� ������ţ���
��3������ʵ�飬���õ�������ƽ��500mL����ƿ���ձ�����ͷ�ιܡ�ҩ���⣬���õ��������� ��
��4������ʱ���ӿ̶ȣ����ʹ��������������Һ��Ũ�� �����ƫ��ƫС����
a��������Ҫ�������ƹ����������
b�������������ƹ��壮
c�����ձ��е���Һע������ƿ��������������ˮϴ���ձ��ڱ�2��3�Σ�ϴ��ҺҲע������ƿ��
d��������������ˮ�ܽ�����õ��������ƹ��壬��ȴ��
e���Ǻ�ƿ�����������µߵ���ҡ�ȣ�
f������������ƿ�м�����ˮ���̶�����1��2cmʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������У�
�ش��������⣺
��1�������
��2�������������ȷ����˳����
��3������ʵ�飬���õ�������ƽ��500mL����ƿ���ձ�����ͷ�ιܡ�ҩ���⣬���õ���������
��4������ʱ���ӿ̶ȣ����ʹ��������������Һ��Ũ��
���㣺��Һ������
ר�⣺ʵ����
��������1����������500mL 0.1mol?L-1������������Һ���������Ƶ����ʵ�������������������������ƾ��и�ʴ�������׳��������
��2������ʵ������IJ��裨������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ������в���˳�������
��3������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ��������������
��4������ʱ��������ƿ�̶��ߣ����¼��������ˮ���ƫС�����Ƶ���Һ���ƫС��
��2������ʵ������IJ��裨������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ������в���˳�������
��3������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ��������������
��4������ʱ��������ƿ�̶��ߣ����¼��������ˮ���ƫС�����Ƶ���Һ���ƫС��
���
�⣺��1������500mL 0.1mol?L-1������������Һ����Ҫ�������Ƶ����ʵ���Ϊ��0.1mol?L-1��0.5L=0.05mol����Ҫ�������Ƶ�����Ϊ��40g/mol��0.05mol=2.0g�������������ƾ��и�ʴ�ԣ����׳�����ʣ����Գ���ʱӦ�÷����ձ��г�����
�ʴ�Ϊ��2.0���ձ���
��2������һ�����ʵ���Ũ�ȵ���Һ������˳���ǣ�������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ�������ȷ�IJ���˳��Ϊ��abdcfe��
�ʴ�Ϊ��abdcfe��
��3������һ�����ʵ���Ũ�ȵ���Һ��Ҫ�������ɣ���ƽ��ҩ�ס���ͷ�ιܡ��ձ�����������500mL����ƿ��
�����õ���δ�ἰ�IJ��������У���������
�ʴ�Ϊ����������
��4������ʱ���ӿ̶ȣ����������ˮ�����������ƿ�̶��ߣ��������Ƶ���Һ���ƫС�����ʹ��������������Һ��Ũ��ƫ��
�ʴ�Ϊ��ƫ��
�ʴ�Ϊ��2.0���ձ���
��2������һ�����ʵ���Ũ�ȵ���Һ������˳���ǣ�������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ�������ȷ�IJ���˳��Ϊ��abdcfe��
�ʴ�Ϊ��abdcfe��
��3������һ�����ʵ���Ũ�ȵ���Һ��Ҫ�������ɣ���ƽ��ҩ�ס���ͷ�ιܡ��ձ�����������500mL����ƿ��
�����õ���δ�ἰ�IJ��������У���������
�ʴ�Ϊ����������
��4������ʱ���ӿ̶ȣ����������ˮ�����������ƿ�̶��ߣ��������Ƶ���Һ���ƫС�����ʹ��������������Һ��Ũ��ƫ��
�ʴ�Ϊ��ƫ��
���������⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ�����ע����������һ�����ʵ���Ũ�ȵ���Һ�ķ������������е��Ѷȵ����⣬���������ǿ�������߿��������������У�ע������ԣ���������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ�����������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
���й�ϵʽ��ȷ���ǣ�������
A����Na2S��Һ�м�ˮϡ�ͣ�
| ||
| B��CH3COONH4��Һ�У�c��NH4+��+c��NH3?H2O��=c��CH3COOH��+c��CH3COO-�� | ||
| C��HCO3- ˮ������ӷ���ʽΪ��HCO3-+H2O?CO32-+H3O+ | ||
| D������ȥCuCl2��Һ�е�����FeCl2��Ӧ����CuO���ٹ��� |
����˵����ȷ���ǣ�������
| A��1g��Ͷ��100gˮ�У���Һ��������Ϊ100g |
| B��1g���汻�����Ľ�����Ͷ��100gˮ�У���Һ��������Ϊ101g |
| C��1g������Ͷ��100gˮ�У���Һ��������Ϊ101g |
| D��1g��������Ͷ��100gˮ�У���Һ��������Ϊ101g |

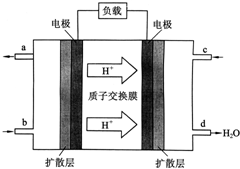 �й���ѧԺ����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ؼ�����ʽ��ѣ��״�ȼ�ϵ�صĹ���ԭ����ͼ��ʾ��
�й���ѧԺ����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ؼ�����ʽ��ѣ��״�ȼ�ϵ�صĹ���ԭ����ͼ��ʾ��