��Ŀ����
5��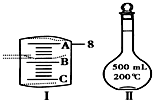 ʵ����Ҫ��NaCl��������500mL 0.2mol•L-1NaCl��Һ���ش��������⣺
ʵ����Ҫ��NaCl��������500mL 0.2mol•L-1NaCl��Һ���ش��������⣺��1��Ӧ����������ƽ��ȡNaCl����5.9g�����ƣ�23 �ȣ�35.5��
��2����ͼ���ʾ10mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����1mL������̶�AΪ8����Ͳ��Һ��������7.2mL��
��3����ʵ������ͼ����ʾ�������������������������Һ��Ũ���к�Ӱ�죿���ƫ�ߡ�����ƫ�͡�����Ӱ�족��
A������ʱ��ˮ�����̶���ƫ�ͣ�
B�����ն���ʱ���ӹ۲�Һ��ƫ�ͣ�
���� ��1������n=cV��m=nM�����㣻
��2����ͲС�̶����£�A��B��C�̶ȼ����1mL���̶�AΪ4����̶�BΪ3��AB��ÿһС��Ϊ0.2mL������Ͳ��Һ������Ϊ3.2mL��
��3������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��� �⣺��1������500mL 0.2mol•L-1NaCl��Һ�����Ȼ��Ƶ����ʵ���Ϊn=cV=0.2mol/L��0.5L=0.1mol������m=nM=0.1mol��58.5g/mol=5.9g���ʴ�Ϊ��5.9��
��2����ͲС�̶����£�A��B��C�̶ȼ����1mL���̶�AΪ8����̶�BΪ7��AB��ÿһС��Ϊ0.2mL������Ͳ��Һ������Ϊ7.2mL���ʴ�Ϊ��7.2��
��3��A������ʱ��ˮ�����̶��ߣ�����Һ���ƫ����Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
B�����ն���ʱ���ӹ۲�Һ�棬����Һ���ƫ��Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

��ϰ��ϵ�д�
 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
16��NA��ʾ�����ӵ�������ֵ������˵����ȷ���ǣ�������
| A�� | 46g�Ҵ��к��й��ۼ�����ĿΪ7NA | |
| B�� | 50ml 2mol•L-1NaClO��Һ��ClO-��ĿΪ0.1NA | |
| C�� | ��״���£�5.6gFe��������Ũ�����ַ�Ӧ��ת�Ƶ�����Ϊ0.2NA | |
| D�� | ���³�ѹ�£�4.4g��CO2��N2O��ɵĻ����������ԭ������Ϊ0.3NA |
10��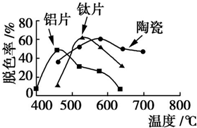 �ڲ�ͬ�����壨��Ƭ����Ƭ���մɣ������Ʊ�TiO2��Ĥ��̽����ͬ������TiO2��Ĥ���ʹ������ɫ��Ч����ÿ����20minȡһ������ʵ������ͼ��ʾ������˵����ȷ���ǣ�������
�ڲ�ͬ�����壨��Ƭ����Ƭ���մɣ������Ʊ�TiO2��Ĥ��̽����ͬ������TiO2��Ĥ���ʹ������ɫ��Ч����ÿ����20minȡһ������ʵ������ͼ��ʾ������˵����ȷ���ǣ�������
 �ڲ�ͬ�����壨��Ƭ����Ƭ���մɣ������Ʊ�TiO2��Ĥ��̽����ͬ������TiO2��Ĥ���ʹ������ɫ��Ч����ÿ����20minȡһ������ʵ������ͼ��ʾ������˵����ȷ���ǣ�������
�ڲ�ͬ�����壨��Ƭ����Ƭ���մɣ������Ʊ�TiO2��Ĥ��̽����ͬ������TiO2��Ĥ���ʹ������ɫ��Ч����ÿ����20minȡһ������ʵ������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��ͬ���壬���ۺ����¶�һ������Ƭ����Ĺ��������� | |
| B�� | ���ۺ������壬�������������¶ȵ����߶����� | |
| C�� | Լ��520��ʱ����Ƭ����Ĺ��������� | |
| D�� | ��ͬ���壬TiO2��Ĥ�Ĺ��������ͬ |
17������ʵ������Ӧ�����ַ�������ȷ���ǣ�������
| ѡ�� | Ŀ�� | ����һ | ������ |
| A | ��ȥľ̿���е�����ͭ | ������ϡ���ᣬ���� | �ڿ����г��ȼ�� |
| B | ����NaCl��CaCO3�Ļ���� | �ܽ⣬���ˣ�������Һ | ������ϡ���ᣬ���� |
| C | ����ϡ�����ϡ����������Һ | �ֱ����Һ��pH | ȡ������FeCl3��Һ |
| D | ����NH4Cl��NH4NO3 | ȡ������ˮ�ܽ⣬�۲� | ȡ��������ʯ����ĥ |
| A�� | A | B�� | B | C�� | C | D�� | D |
14����ͨ�������£����и������ʵ�����������ȷ���ǣ�������
| A�� | �ܶȣ�����ˮ�����Ȼ�̼ | B�� | ��ԭ�ԣ�HF��HCl��HBr��HI | ||
| C�� | ���ȶ��ԣ�HF��H2O��NH3 | D�� | ˮ���ԣ�HCl��NH3��SO2 |
15�������£����и�������һ���ܴ���������ǣ�������
| A�� | pH��7����Һ�У�Na+��Mg2+��S042-��I- | |
| B�� | ʹ������Һ������Һ�У�Al3+��Cu2+��N03-��SO42- | |
| C�� | ��ˮ�������c��H+��=l.0��10-13mol•L-1����Һ�У�Na+��C032-��SO32-��Clһ | |
| D�� | �������ܲ�������H2����Һ�У�Na+��NH4+��N03-��Cl- |
 ��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������980mL��0.1mol/L��ϡ���ᣮ�ɹ�ѡ�õ������У�
��ͼ��ijѧУʵ���Ҵӻ�ѧ�Լ��̵���ص������Լ���ǩ�ϵIJ������ݣ����ø�Ũ��������980mL��0.1mol/L��ϡ���ᣮ�ɹ�ѡ�õ������У�