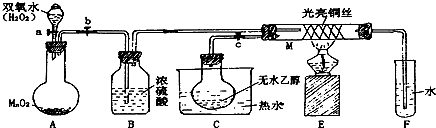
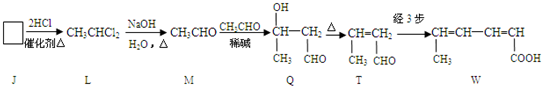
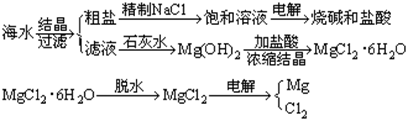
��Ŀ����
A��B��C��D������ͬһ��Ԫ�أ�����֮���ת����ϵ����Ӧ���������������ȥ����A
B
D
����д���пհף�
��1����A��D��ˮ��Һ����ʹʪ�����ɫʯ����ֽ��죬��DΪ����д��ѧʽ�� ��
��2����AΪ���壬��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ������D��ϡ��Һ��ʹʪ�����ɫʯ����ֽ��죮��ҵ�Ϻϳ�A�Ļ�ѧ����ʽΪ ��
��3����AΪ������Ԫ���γɵķǽ������ʣ���A������ ����3�ֲ�ͬ���ʵĻ�ѧʽ����
| O2 |
| H2O |
����д���пհף�
��1����A��D��ˮ��Һ����ʹʪ�����ɫʯ����ֽ��죬��DΪ����д��ѧʽ��
��2����AΪ���壬��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ������D��ϡ��Һ��ʹʪ�����ɫʯ����ֽ��죮��ҵ�Ϻϳ�A�Ļ�ѧ����ʽΪ
��3����AΪ������Ԫ���γɵķǽ������ʣ���A������
���㣺������ƶ�
ר�⣺�ƶ���
��������1��A��D��ˮ��Һ����ʹʪ�����ɫʯ����ֽ��죬Ϊ�������ʣ����ת����ϵ����AΪH2S��BΪSO2��CΪSO2��DΪH2SO4��
��2����AΪ���壬��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ��������AΪNH3��BΪNO��CΪNO2��DΪHNO3��
��3����AΪ������Ԫ���γɵķǽ������ʣ���A������̼����N2��
��2����AΪ���壬��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ��������AΪNH3��BΪNO��CΪNO2��DΪHNO3��
��3����AΪ������Ԫ���γɵķǽ������ʣ���A������̼����N2��
���
�⣺��1��A��D��ˮ��Һ����ʹʪ�����ɫʯ����ֽ��죬Ϊ�������ʣ����ת����ϵ����AΪH2S��BΪSO2��CΪSO2��DΪH2SO4���ʴ�Ϊ��H2SO4��
��2����AΪ���壬��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ��������AΪNH3��BΪNO��CΪNO2��DΪHNO3����ҵ�Ϻϳ�A�Ļ�ѧ����ʽΪ��N2+3H2
2NH3���ʴ�Ϊ��N2+3H2
2NH3��
��3����AΪǰ18��Ԫ���γɵķǽ������ʣ���AΪ̼����N2����ת����ϵ���ʴ�Ϊ��C��S��N2��
��2����AΪ���壬��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ��������AΪNH3��BΪNO��CΪNO2��DΪHNO3����ҵ�Ϻϳ�A�Ļ�ѧ����ʽΪ��N2+3H2
| ||
| ���¸�ѹ |
| ||
| ���¸�ѹ |
��3����AΪǰ18��Ԫ���γɵķǽ������ʣ���AΪ̼����N2����ת����ϵ���ʴ�Ϊ��C��S��N2��
���������⿼�������ƶϣ��Ѷ��еȣ���Ҫѧ����������Ԫ�ػ�����֪ʶ��ע��������ѧ��������������Ӧ��

��ϰ��ϵ�д�
�����Ŀ
��֪25��ʱ�ϳɰ���Ӧ���Ȼ�ѧ����ʽΪ��N2��g��+3H2��g���T2NH3��g����H=-92.4kJ/mol��һ��ѹǿ�£���10L���ܱ����������1mol N2��3mol H2���ﵽƽ���NH3���������Ϊ60%������ͬ�����£���ͬһ�ܱ�������ij���2mol NH3����������ЧӦΪa����ʱNH3��ת����Ϊ�����������ж���ȷ���ǣ�������
| A��a=-23.1kJ��=40% |
| B��a=-23.1kJ ��=25% |
| C��a=+23.1kJ��=40% |
| D��a=+23.1kJ ��=25% |
��֤�������Ѳ���ˮ����Լ������еģ�������
| A�����۵⻯����Һ | B��������Һ |
| C����ˮ | D���⻯����Һ |
��ˮƿ���������õĻ�ԭ���ǣ�������
| A���������� | B��������Һ |
| C�������� | D������ |
���б仯��ͨ��һ����ѧ��Ӧʵ�ֵ��ǣ�������
| A��Na2O2��Na |
| B��Al2O3��Al��OH��3 |
| C��SiO2��H2SiO3 |
| D��Fe��OH��2��Fe��OH��3 |