��Ŀ����
���л������HCl ��NaOH ��CH3COOH ��NH3?H2O ��CH3COONa ��NH4Cl��1������������ʵ��� ����Һ�ʼ��Ե��� ������ţ���
��2��������0.01mol/L HCl��Һ��PH= ��pH=11��CH3COONa��Һ����ˮ���������c��OH-��= ��
��3�������ӷ���ʽ��ʾCH3COONa��Һ�ʼ��Ե�ԭ�� ������Һ������Ũ�Ȱ��ɴ�С��˳��Ϊ ��
��4������pH�������HCl��CH3COOH�ֱ�ϡ��m����n����ϡ�ͺ�����Һ��PH����ȣ���m n ������ڡ����ڡ�С�ڡ�����
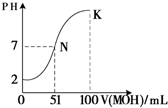
��5�������£���100mL 0.01mol?L-1HA��Һ��μ���0.02mol?L-1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ����ش��������⣺
����ͼ����Ϣ��֪HAΪ �ᣨ�ǿ������������
��K���Ӧ����Һ�У�c��M+��+c��MOH��= mol?L-1��

���𰸡���������1����ˮ��Һ�������״̬��ֻ�в��ֵ���ĵ������������ʣ����ǿ������������Һ���ʼ��ԣ�
��2��pH=- �������ƴٽ�ˮ���룬����������Ũ�Ƚ��ˮ�����ӻ�������������������Ũ�ȣ�
�������ƴٽ�ˮ���룬����������Ũ�Ƚ��ˮ�����ӻ�������������������Ũ�ȣ�
��3����������ǿ�������Σ����������ˮ���ʹ����Һ�ʼ��ԣ����ݵ���غ�ȷ������Ũ�ȴ�С��
��4��������������ʣ��Ȼ�����ǿ����ʣ�������ڵ���ƽ�⣬�Ȼ��ⲻ���ڵ���ƽ�⣬����pH�Ĵ��������ϡ�ͺ���Һ��pH��Ȼ��ȣ������ϡ�͵ı����������
��5���ٸ������Ũ�Ⱥ���Һ��PH�ж����ǿ����
�ڸ��������غ���㣮
����⣺��1����ˮ��Һ�������״̬��ֻ�в��ֵ���ĵ������������ʣ�����������ʵ���һˮ�ϰ��ʹ��ᣬ���ǿ������������Һ���ʼ��ԣ��������ƺͰ�ˮ����������Һ���ʼ��ԣ���ѡ���ۢܡ��ڢܢݣ�
��2��pH=- =-lg0.01=2�������ƴٽ�ˮ���룬��������Һ��������Ũ��Ϊ10-11mol/L������ˮ�����ӻ�����֪������������Ũ�ȵ���10-3 mol/L��
=-lg0.01=2�������ƴٽ�ˮ���룬��������Һ��������Ũ��Ϊ10-11mol/L������ˮ�����ӻ�����֪������������Ũ�ȵ���10-3 mol/L��
�ʴ�Ϊ��2��10-3 mol/L��
��3����������ǿ�������Σ����������ˮ�����ɴ��ᣬ������Һ������������Ũ�ȴ���������Ũ�ȶ�ʹ����Һ�ʼ��ԣ�ˮ�����ӷ���ʽΪ��CH3COO?+H2O?CH3COOH+OH?����Һ�ʼ��ԣ�����c��OH-����c��H+������Һ�д��ڵ���غ㣬c��Na+��+c��H+��=c��CH3COO?��+c��OH-������c��Na+����c��CH3COO?������������Ũ�ȴ�С˳����c��Na+����c��CH3COO?����c��OH-����c��H+����
�ʴ�Ϊ��CH3COO?+H2O?CH3COOH+OH?��c��Na+����c��CH3COO?����c��OH-����c��H+����
��4��������������ʣ��Ȼ�����ǿ����ʣ�������ڵ���ƽ�⣬�Ȼ��ⲻ���ڵ���ƽ�⣬����pH�Ĵ��������ϡ�ͺ���Һ��pH��Ȼ��ȣ�������ϡ�͵ı���С�ڴ��ᣬ�ʴ�Ϊ��С�ڣ�
��5���ٸ���ͼ��֪��0.01mol?L-1HA��Һ��PH=2��������Ũ�ȵ�����Ũ�ȣ����Ը�����ǿ�ᣬ�ʴ�Ϊ��ǿ��
��K��ʱ�������MOH�����ʵ���=0.02mol?L-1×0.1L=0.002mol�������Һ�������0.2L�����������غ�֪��c��M+��+c��MOH��= =0.01mol/L���ʴ�Ϊ��0.01��
=0.01mol/L���ʴ�Ϊ��0.01��
���������⿼����������ʵĵ��롢�����ˮ�⡢��Һ��ϡ�͵�֪ʶ�㣬���õ���غ㡢�����غ���������ɣ��Ѷ��еȣ�
��2��pH=-
 �������ƴٽ�ˮ���룬����������Ũ�Ƚ��ˮ�����ӻ�������������������Ũ�ȣ�
�������ƴٽ�ˮ���룬����������Ũ�Ƚ��ˮ�����ӻ�������������������Ũ�ȣ���3����������ǿ�������Σ����������ˮ���ʹ����Һ�ʼ��ԣ����ݵ���غ�ȷ������Ũ�ȴ�С��
��4��������������ʣ��Ȼ�����ǿ����ʣ�������ڵ���ƽ�⣬�Ȼ��ⲻ���ڵ���ƽ�⣬����pH�Ĵ��������ϡ�ͺ���Һ��pH��Ȼ��ȣ������ϡ�͵ı����������
��5���ٸ������Ũ�Ⱥ���Һ��PH�ж����ǿ����
�ڸ��������غ���㣮
����⣺��1����ˮ��Һ�������״̬��ֻ�в��ֵ���ĵ������������ʣ�����������ʵ���һˮ�ϰ��ʹ��ᣬ���ǿ������������Һ���ʼ��ԣ��������ƺͰ�ˮ����������Һ���ʼ��ԣ���ѡ���ۢܡ��ڢܢݣ�
��2��pH=-
 =-lg0.01=2�������ƴٽ�ˮ���룬��������Һ��������Ũ��Ϊ10-11mol/L������ˮ�����ӻ�����֪������������Ũ�ȵ���10-3 mol/L��
=-lg0.01=2�������ƴٽ�ˮ���룬��������Һ��������Ũ��Ϊ10-11mol/L������ˮ�����ӻ�����֪������������Ũ�ȵ���10-3 mol/L���ʴ�Ϊ��2��10-3 mol/L��
��3����������ǿ�������Σ����������ˮ�����ɴ��ᣬ������Һ������������Ũ�ȴ���������Ũ�ȶ�ʹ����Һ�ʼ��ԣ�ˮ�����ӷ���ʽΪ��CH3COO?+H2O?CH3COOH+OH?����Һ�ʼ��ԣ�����c��OH-����c��H+������Һ�д��ڵ���غ㣬c��Na+��+c��H+��=c��CH3COO?��+c��OH-������c��Na+����c��CH3COO?������������Ũ�ȴ�С˳����c��Na+����c��CH3COO?����c��OH-����c��H+����
�ʴ�Ϊ��CH3COO?+H2O?CH3COOH+OH?��c��Na+����c��CH3COO?����c��OH-����c��H+����
��4��������������ʣ��Ȼ�����ǿ����ʣ�������ڵ���ƽ�⣬�Ȼ��ⲻ���ڵ���ƽ�⣬����pH�Ĵ��������ϡ�ͺ���Һ��pH��Ȼ��ȣ�������ϡ�͵ı���С�ڴ��ᣬ�ʴ�Ϊ��С�ڣ�
��5���ٸ���ͼ��֪��0.01mol?L-1HA��Һ��PH=2��������Ũ�ȵ�����Ũ�ȣ����Ը�����ǿ�ᣬ�ʴ�Ϊ��ǿ��
��K��ʱ�������MOH�����ʵ���=0.02mol?L-1×0.1L=0.002mol�������Һ�������0.2L�����������غ�֪��c��M+��+c��MOH��=
 =0.01mol/L���ʴ�Ϊ��0.01��
=0.01mol/L���ʴ�Ϊ��0.01�����������⿼����������ʵĵ��롢�����ˮ�⡢��Һ��ϡ�͵�֪ʶ�㣬���õ���غ㡢�����غ���������ɣ��Ѷ��еȣ�

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д�
�����Ŀ
 ��������HCl ��NaOH ��CH3COOH ��NH3?H2O ��CH3COONa ��NH4Cl
��������HCl ��NaOH ��CH3COOH ��NH3?H2O ��CH3COONa ��NH4Cl