��Ŀ����
��������HCl ��NaOH ��CH3COOH ��NH3?H2O ��CH3COONa ��NH4Cl
��1������������ʵ���______����Һ�ʼ��Ե���______������ţ���
��2��������0.01mol/L HCl��Һ��PH=______��pH=11��CH3COONa��Һ����ˮ���������c��OH-��=______��
��3�������ӷ���ʽ��ʾCH3COONa��Һ�ʼ��Ե�ԭ��______������Һ������Ũ�Ȱ��ɴ�С��˳��Ϊ______��
��4������pH�������HCl��CH3COOH�ֱ�ϡ��m����n����ϡ�ͺ�����Һ��PH����ȣ���m______n ������ڡ����ڡ�С�ڡ�����
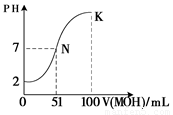
��5�������£���100mL 0.01mol?L-1HA��Һ��μ���0.02mol?L-1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ����ش��������⣺
����ͼ����Ϣ��֪HAΪ______�ᣨ�ǿ������������
��K���Ӧ����Һ�У�c��M+��+c��MOH��=______mol?L-1��
��1������������ʵ���______����Һ�ʼ��Ե���______������ţ���
��2��������0.01mol/L HCl��Һ��PH=______��pH=11��CH3COONa��Һ����ˮ���������c��OH-��=______��
��3�������ӷ���ʽ��ʾCH3COONa��Һ�ʼ��Ե�ԭ��______������Һ������Ũ�Ȱ��ɴ�С��˳��Ϊ______��
��4������pH�������HCl��CH3COOH�ֱ�ϡ��m����n����ϡ�ͺ�����Һ��PH����ȣ���m______n ������ڡ����ڡ�С�ڡ�����
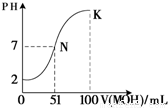
��5�������£���100mL 0.01mol?L-1HA��Һ��μ���0.02mol?L-1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ����ش��������⣺
����ͼ����Ϣ��֪HAΪ______�ᣨ�ǿ������������
��K���Ӧ����Һ�У�c��M+��+c��MOH��=______mol?L-1��

��1����ˮ��Һ�������״̬��ֻ�в��ֵ���ĵ������������ʣ�����������ʵ���һˮ�ϰ��ʹ��ᣬ���ǿ������������Һ���ʼ��ԣ��������ƺͰ�ˮ����������Һ���ʼ��ԣ���ѡ���ۢܡ��ڢܢݣ�
��2��pH=-lgc(H+)=-lg0.01=2�������ƴٽ�ˮ���룬��������Һ��������Ũ��Ϊ10-11mol/L������ˮ�����ӻ�����֪������������Ũ�ȵ���10-3 mol/L��
�ʴ�Ϊ��2��10-3 mol/L��
��3����������ǿ�������Σ����������ˮ�����ɴ��ᣬ������Һ������������Ũ�ȴ���������Ũ�ȶ�ʹ����Һ�ʼ��ԣ�ˮ�����ӷ���ʽΪ��CH3COO?+H2O?CH3COOH+OH?����Һ�ʼ��ԣ�����c��OH-����c��H+������Һ�д��ڵ���غ㣬c��Na+��+c��H+��=c��CH3COO?��+c��OH-������c��Na+����c��CH3COO?������������Ũ�ȴ�С˳����c��Na+����c��CH3COO?����c��OH-����c��H+����
�ʴ�Ϊ��CH3COO?+H2O?CH3COOH+OH?��c��Na+����c��CH3COO?����c��OH-����c��H+����
��4��������������ʣ��Ȼ�����ǿ����ʣ�������ڵ���ƽ�⣬�Ȼ��ⲻ���ڵ���ƽ�⣬����pH�Ĵ��������ϡ�ͺ���Һ��pH��Ȼ��ȣ�������ϡ�͵ı���С�ڴ��ᣬ�ʴ�Ϊ��С�ڣ�
��5���ٸ���ͼ��֪��0.01mol?L-1HA��Һ��PH=2��������Ũ�ȵ�����Ũ�ȣ����Ը�����ǿ�ᣬ�ʴ�Ϊ��ǿ��
��K��ʱ�������MOH�����ʵ���=0.02mol?L-1��0.1L=0.002mol�������Һ�������0.2L�����������غ�֪��c��M+��+c��MOH��=
=0.01mol/L���ʴ�Ϊ��0.01��
��2��pH=-lgc(H+)=-lg0.01=2�������ƴٽ�ˮ���룬��������Һ��������Ũ��Ϊ10-11mol/L������ˮ�����ӻ�����֪������������Ũ�ȵ���10-3 mol/L��
�ʴ�Ϊ��2��10-3 mol/L��
��3����������ǿ�������Σ����������ˮ�����ɴ��ᣬ������Һ������������Ũ�ȴ���������Ũ�ȶ�ʹ����Һ�ʼ��ԣ�ˮ�����ӷ���ʽΪ��CH3COO?+H2O?CH3COOH+OH?����Һ�ʼ��ԣ�����c��OH-����c��H+������Һ�д��ڵ���غ㣬c��Na+��+c��H+��=c��CH3COO?��+c��OH-������c��Na+����c��CH3COO?������������Ũ�ȴ�С˳����c��Na+����c��CH3COO?����c��OH-����c��H+����
�ʴ�Ϊ��CH3COO?+H2O?CH3COOH+OH?��c��Na+����c��CH3COO?����c��OH-����c��H+����
��4��������������ʣ��Ȼ�����ǿ����ʣ�������ڵ���ƽ�⣬�Ȼ��ⲻ���ڵ���ƽ�⣬����pH�Ĵ��������ϡ�ͺ���Һ��pH��Ȼ��ȣ�������ϡ�͵ı���С�ڴ��ᣬ�ʴ�Ϊ��С�ڣ�
��5���ٸ���ͼ��֪��0.01mol?L-1HA��Һ��PH=2��������Ũ�ȵ�����Ũ�ȣ����Ը�����ǿ�ᣬ�ʴ�Ϊ��ǿ��
��K��ʱ�������MOH�����ʵ���=0.02mol?L-1��0.1L=0.002mol�������Һ�������0.2L�����������غ�֪��c��M+��+c��MOH��=
| 0.002mol |
| 0.2L |

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��������HCl ��NaOH ��CH3COOH ��NH3?H2O ��CH3COONa ��NH4Cl
��������HCl ��NaOH ��CH3COOH ��NH3?H2O ��CH3COONa ��NH4Cl