��Ŀ����
2��H2��I2��һ���������ܷ�����Ӧ��H2��g��+I2��g��?2HI��g����1molH2��ȫ��Ӧ�ų�akJ��������֪��
 ��a��b��c�������㣩
��a��b��c�������㣩����˵������ȷ���ǣ�������
| A�� | ��Ӧ�������������������������� | |
| B�� | �Ͽ�1molH-H����1molI-I�������������ڶϿ�2molH-I���������� | |
| C�� | �Ͽ�2molH-I����������ԼΪ��c+b+a��kJ | |
| D�� | ���ܱ������м���2molH2��2molI2����ַ�Ӧ�ų���������С2akJ |
���� A.1molH2��ȫ��Ӧ�ų�akJ��������֪Ϊ���ȷ�Ӧ��
B���ʱ���ѻ�ѧ�����յ�������ȥ�ɼ��ͷŵ�������
C����Ͽ�2molH-I����������Ϊx������Ϣ��֪��b+c-2x=-a��
D�����ʵ��������������ȣ��Ҹ÷�ӦΪ���淴Ӧ��
��� �⣺A.1molH2��ȫ��Ӧ�ų�akJ��������֪Ϊ���ȷ�Ӧ����Ӧ������������������������������A��ȷ��
B���ʱ���ѻ�ѧ�����յ�������ȥ�ɼ��ͷŵ��������÷�ӦΪ���ȷ�Ӧ����Ͽ�1molH-H����1molI-I����������С�ڶϿ�2molH-I��������������B����
C����Ͽ�2molH-I����������Ϊx������Ϣ��֪��b+c-x=-a��������ԼΪ��c+b+a��kJ����C��ȷ��
D�����ʵ��������������ȣ��Ҹ÷�ӦΪ���淴Ӧ�����ַ�Ӧ�ų�������С��2akJ����D��ȷ��
��ѡB��
���� ���⿼�鷴Ӧ�����ʱ䣬Ϊ��Ƶ���㣬���շ�Ӧ�������仯���������ʱ�Ĺ�ϵΪ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע��ѡ��DΪ�״��㣬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
13����������ϩ�����ǣ�������
| A�� | CH3CH2CH3 | B�� | CH2=CHCH3 | C�� | CH2=CHCH2Cl | D�� | CH2=CHCOOH |
10��ij��ҵ��ˮ�н����������е�5�֣��Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1mol•L-1��
��̽����ˮ����ɣ���������ʵ�飺
I���ò�˿պȡ������ҺҺ���ڻ��������գ�����ɫ���棨����ɫ�ܲ����۲죩��
��ȡ����Һ������KSCN��Һ�����Ա仯��
����ȡ��Һ�����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ�Μ[������Һ������������䣮
������������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɣ�
��1������I�����жϣ���Һ��һ�������е���������K+��Fe3+ ��д���ӷ��ţ���
��2�����������ú���ɫ����ͨ��ˮ�У��������ɫ���������Ļ�ѧ����ʽΪ��3NO2+H2O�T2HNO3+NO��
��3��ԭ��Һ��������������Fe2+��Cu2+����������Cl-��NO3-��SO42-����д���ӷ��ţ�
| ������ | K+ Cu2+ Fe3+ Al3+ Fe2+ |
| ������ | Cl- CO32- NO3- SO42- SiO32- |
I���ò�˿պȡ������ҺҺ���ڻ��������գ�����ɫ���棨����ɫ�ܲ����۲죩��
��ȡ����Һ������KSCN��Һ�����Ա仯��
����ȡ��Һ�����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ�Μ[������Һ������������䣮
������������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɣ�
��1������I�����жϣ���Һ��һ�������е���������K+��Fe3+ ��д���ӷ��ţ���
��2�����������ú���ɫ����ͨ��ˮ�У��������ɫ���������Ļ�ѧ����ʽΪ��3NO2+H2O�T2HNO3+NO��
��3��ԭ��Һ��������������Fe2+��Cu2+����������Cl-��NO3-��SO42-����д���ӷ��ţ�
17��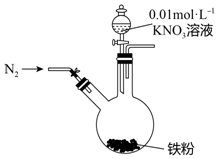 ijͬѧ�����������������ԭNO3-�ѳ�����ˮ�������Ρ���������Ϻ���������װ��̽��������KNO3��Һ�ķ�Ӧ��ʵ�鲽�輰�������£�
ijͬѧ�����������������ԭNO3-�ѳ�����ˮ�������Ρ���������Ϻ���������װ��̽��������KNO3��Һ�ķ�Ӧ��ʵ�鲽�輰�������£�
��1��ͨ��N2�����ֺ�����Ӧ����N2��Χ�н��е�ʵ��Ŀ���Ƿ�ֹ�����е�O2��Fe��NO3-��Ӧ�ĸ��ţ�����Ӱ�췴Ӧ������жϣ�
��2����ɫ������Fe��OH��2���û�ѧ����ʽ�������Ϊ���ɫ��ԭ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��3��Ϊ��̽���Һ�ijɷ֣���ͬѧ��һ�����������ʵ�飺
��i����������ʵ���������ж���Һ�д���NH4+��Fe2+��NO3-���ӣ�
��ii������2�еμ�ϡ�������Һ����dz��ɫ��ɺ�ɫ���������ӷ���ʽ������ԭ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��
 ijͬѧ�����������������ԭNO3-�ѳ�����ˮ�������Ρ���������Ϻ���������װ��̽��������KNO3��Һ�ķ�Ӧ��ʵ�鲽�輰�������£�
ijͬѧ�����������������ԭNO3-�ѳ�����ˮ�������Ρ���������Ϻ���������װ��̽��������KNO3��Һ�ķ�Ӧ��ʵ�鲽�輰�������£�| ʵ�鲽�� | ʵ������ |
| 1�����ɼУ�����ͨ��N2 | |
| 2������pHΪ2.5��0.01mol/L����KNO3��Һ100mL | ���۲����ܽ⣬��Һ��dz��ɫ�� ���۲����ܽ��ʣ�����۱������������ɫ���ʸ��ţ� |
| 3����Ӧֹͣ�ε���������Բ����ƿȡ�� | ��ƿ���������ɫû�з����仯�� |
| 4��ʣ�������� | ����İ�ɫ���ʱ�Ϊ���ɫ�� |
��2����ɫ������Fe��OH��2���û�ѧ����ʽ�������Ϊ���ɫ��ԭ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��3��Ϊ��̽���Һ�ijɷ֣���ͬѧ��һ�����������ʵ�飺
| ʵ�鲽�� | ʵ������ |
| 1��ȡ������Һ���Թ��У������м���KSCN��Һ | ��ҺҺ�ޱ仯 |
| 2����������Һ��Ϊ���ݣ�һ���е�����������һ���еμ�ϡ���� | ������Һ����Ϊ��ɫ |
| 3����ȡ������Һ���Թ��У������м���ŨNaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ�� | ���������ɣ�������ʹ��ɫʯ����ֽ������ |
��ii������2�еμ�ϡ�������Һ����dz��ɫ��ɺ�ɫ���������ӷ���ʽ������ԭ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��
7��ijͬѧ����ƬA��̼��B�õ������Ӻ����ʳ��ˮ��ģ��˵�������̣�����������������ǣ�������


| A�� | �������е�����������������ΪA����B | |
| B�� | ��Һ�е�O2�ڵ缫B�õ��ӣ��缫B����������ǿ | |
| C�� | �缫A�ϵĵ缫��ӦʽΪFe-2e-=Fe2+ | |
| D�� | ��Һ�е�Na+��缫B�����ƶ� |
14�����и�װ���ܹ�����ԭ��ص��ǣ�������
| A�� |  | B�� |  | ||
| C�� |  | D�� |  |
12����NAΪ�����ӵ���������ֵ�������й�������ȷ���ǣ�������
| A�� | 1 mol�Ҵ���������������ȩ��ת�Ƶĵ�����Ϊ4NA | |
| B�� | 30g��14C2H2��C18O��ɵĻ�������к��е�������Ϊ14NA | |
| C�� | ��״���£�44.8LSO2������O2��Ӧ���ɵ�SO3������Ϊ2 NA | |
| D�� | 60g SiO2��12g���ʯ�и�����4NA��Si-O����C-C�� |
 ���һЩ�����ﳣ�����뵼�塢���ݼ���ɱ��ҩ�ȣ��ش��������⣺
���һЩ�����ﳣ�����뵼�塢���ݼ���ɱ��ҩ�ȣ��ش��������⣺

 +��2n-1��H2O��
+��2n-1��H2O��