��Ŀ����
6��±������ȡ����Ӧ��ʵ���Ǵ�����ɵ�ԭ����ȡ����±�����е�±ԭ�ӣ����磺CH3Br+OH-����NaOH����CH3OH+Br- ����NaBr�������з�Ӧ�Ļ�ѧ��Ӧ����ʽ�в���ȷ���ǣ�������| A�� | CH3CH2Br+CH3COONa��CH3COOCH2CH3+NaBr | |
| B�� | CH3I+CH3ONa��CH3OCH3+NaI | |
| C�� | CH3CH2Cl+CH3ONa��CH3Cl+CH3CH2ONa | |
| D�� | CH3CH2Cl+CH3CH2ONa����CH3CH2�� 2O+NaCl |
���� ����������Ϣ��±����ȡ����Ӧ��ʵ���Ǵ�����ɵ�ԭ����ȡ��±��ԭ�ӡ����ȸ��������Ӵ�������жϷ�Ӧ���д�����ɵ�ԭ���ţ�Ȼ������ɵ�ԭ����ȡ��±��ԭ�Ӽ��ɣ��ݴ���ɱ��⣮
A��������ɵ�ԭ����Ϊ��CH3COO-��B��������ɵ�ԭ����Ϊ��CH3O-��C��������ɵ�ԭ����Ϊ��CH3O-��D��������ɵ�ԭ����Ϊ��CH3CH2O-��
��� �⣺A��CH3COONa�д�����ɵ�ԭ����ΪCH3COO-��CH3COO-ȡ����ԭ�ӣ���Ӧ�Ļ�ѧ����ʽΪ��CH3CH2Br+CH3COONa��NaBr+CH3COOCH2CH3����A��ȷ��
B��CH3ONa�д�����ɵ�ԭ����ΪCH3O-��CH3O-ȡ����ԭ�ӣ���Ӧ�Ļ�ѧ����ʽΪ��CH3I+CH3ONa��NaI+CH3OCH3����B��ȷ��
C��CH3ONa�д�����ɵ�ԭ����ΪCH3O-��CH3O-ȡ����ԭ�ӣ���Ӧ�Ļ�ѧ����ʽΪ��CH3CH2Cl+CH3ONa��NaCl+CH3CH2OCH3����C����
D��CH3CH2ONa�д�����ɵ�ԭ����Ϊ��CH3CH2O-��CH3CH2O-ȡ����ԭ�ӣ���Ӧ�Ļ�ѧ����ʽΪ��CH3CH2Cl+CH3CH2ONa����CH3CH2��2O+NaCl����D��ȷ��
��ѡC��
���� ���⿼����ȡ����Ӧ����Ŀ�ѶȲ���������Ǻ����������з�Ӧ��ԭ����������ض�ѧ������֪ʶ��ѵ���ͼ��飬����������ѧ�������������������ѧ��������û���֪ʶ���ʵ�������������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | NaF | B�� | MgI2 | C�� | NaI | D�� | KBr |
| A�� | C2H4 | B�� | C2H2 | C�� | C6H6������ | D�� | CH3-CH=CH2 |
| A�� | 2C2H2��g��+5O2��g���T4CO2��g��+2H2O��l����H=-2b kJ/mol | |
| B�� | 2C2H2��g��+5O2��g���T4CO2��g��+2H2O��l����H=-4b kJ/mol | |
| C�� | C2H2��g��+O2��g���T2CO2��g��+H2O��l����H=+2b kJ/mol | |
| D�� | 2C2H2��g��+5O2��g���T4CO2��g��+2H2O��l����H=-$\frac{b}{a}$kJ/mol |

 ��C
��C ��D
��D ��
�� ��
�� ��
�� ��
�� C7H16��ͬ���칹���о��С�����̼ԭ�ӡ�����2�֣�д������һ�ֵ�����3-�����飨��2��3-�������飩��
C7H16��ͬ���칹���о��С�����̼ԭ�ӡ�����2�֣�д������һ�ֵ�����3-�����飨��2��3-�������飩��

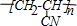 ����Ӧ�������ͷֱ�Ϊ�ӳɷ�Ӧ���Ӿ۷�Ӧ��
����Ӧ�������ͷֱ�Ϊ�ӳɷ�Ӧ���Ӿ۷�Ӧ��