��Ŀ����
��һ��ϡ��Һ�������������֣���Һ��ɫ�����壬���п��ܺ���SO42-��Na+��CO32-��H+��NO3-��NH4+��Cl- �������е������֣�Ȼ������������ʵ�飬��ȷ����Щ�����Ƿ�������ڣ�
����pH��ֽ����Һ��pH����ֽ�Ժ�ɫ��
��ȡ2������Һ�����Ȼ�����ϡ������м��飬��������˰�ɫ������
�۶Ԣ������û�����־��ú�ȡ�ϲ���ҹ����������Һ��ϡ������飬����������˰�ɫ������
����ȡ2������Һ��������������������Һ�����ȣ�û�в�����ʹʪ���ɫʯ����ֽ���������壮
�Իش��������⣺
��1��ԭ��Һ��һ�����ڵ�������
��2��ԭ��Һ��һ�������ڵ�������
��3��������������ԭ��Һ�л����ܿ϶��Ƿ���ڵ��������� ����������ӷ����� ��
����pH��ֽ����Һ��pH����ֽ�Ժ�ɫ��
��ȡ2������Һ�����Ȼ�����ϡ������м��飬��������˰�ɫ������
�۶Ԣ������û�����־��ú�ȡ�ϲ���ҹ����������Һ��ϡ������飬����������˰�ɫ������
����ȡ2������Һ��������������������Һ�����ȣ�û�в�����ʹʪ���ɫʯ����ֽ���������壮
�Իش��������⣺
��1��ԭ��Һ��һ�����ڵ�������
��2��ԭ��Һ��һ�������ڵ�������
��3��������������ԭ��Һ�л����ܿ϶��Ƿ���ڵ���������
���㣺�������ӵļ��鷽��
ר�⣺
����������pH��ֽ����Һ��pH����ֽ�Ժ�ɫ��˵����Һ�����ԣ���H+���ӷ�Ӧ�����Ӳ��ܹ��棬
��ȡ2mL������Һ�����������Ȼ�����Һ��ϡ���ᣬ��������˲�����ϡ����İ�ɫ�������ó���ΪBaSO4������˵����Һ�к���SO42-���ӣ�
��ȡ���е��ϲ���Һ����ʱ�����������ӣ�������������Һ������������˲�����ϡ����İ�ɫ����������˵����Һ�к���Cl-���ӣ�
����ȡ2������Һ��������������������Һ�����ȣ�û�в�����ʹʪ���ɫʯ����ֽ���������壬һ��û��NH4+���ݴ˷�����ɣ�
��ȡ2mL������Һ�����������Ȼ�����Һ��ϡ���ᣬ��������˲�����ϡ����İ�ɫ�������ó���ΪBaSO4������˵����Һ�к���SO42-���ӣ�
��ȡ���е��ϲ���Һ����ʱ�����������ӣ�������������Һ������������˲�����ϡ����İ�ɫ����������˵����Һ�к���Cl-���ӣ�
����ȡ2������Һ��������������������Һ�����ȣ�û�в�����ʹʪ���ɫʯ����ֽ���������壬һ��û��NH4+���ݴ˷�����ɣ�
���
�⣺����pH��ֽ����Һ��pH����ֽ�Ժ�ɫ��˵����Һ�����ԣ�˵����Һ�����ԣ�CO32-��H+���ӷ�Ӧ�����ܴ������棬��һ��������CO32-��
�ڼ��������Ȼ�����ϡ������Һ����������˲�����ϡ����İ�ɫ�������ó���ΪBaSO4��˵����Һ�к���SO42-���ӣ�
��ȡ���е��ϲ���Һ����ʱ���������ӣ�������������Һ������������˲�����ϡ����İ�ɫ����������˵����Һ�к���Cl-���ӣ�
����ȡ2������Һ��������������������Һ�����ȣ�û�в�����ʹʪ���ɫʯ����ֽ���������壬һ��û��NH4+��
��1��ԭ��Һ��һ�����ڵ�������H+��SO42-���ʴ�Ϊ��H+��SO42-��
��2��ԭ��Һ��һ��û��CO32-��NH4+���ʴ�Ϊ��CO32-��NH4+��
��3��ԭ��Һ�л����ܿ϶��Ƿ���ڵ���������Na+������������ӷ�������ɫ��Ӧ����ȡһ���ɾ��IJ�˿��պȡ��������Һ���ڻ������գ��۲��Ƿ���ֻ�ɫ���ʴ�Ϊ��ȡһ���ɾ��IJ�˿��պȡ��������Һ���ڻ������գ��۲��Ƿ���ֻ�ɫ��
�ʴ�Ϊ��Na+��ȡһ���ɾ��IJ�˿��պȡ��������Һ���ڻ������գ��۲��Ƿ���ֻ�ɫ��
�ڼ��������Ȼ�����ϡ������Һ����������˲�����ϡ����İ�ɫ�������ó���ΪBaSO4��˵����Һ�к���SO42-���ӣ�
��ȡ���е��ϲ���Һ����ʱ���������ӣ�������������Һ������������˲�����ϡ����İ�ɫ����������˵����Һ�к���Cl-���ӣ�
����ȡ2������Һ��������������������Һ�����ȣ�û�в�����ʹʪ���ɫʯ����ֽ���������壬һ��û��NH4+��
��1��ԭ��Һ��һ�����ڵ�������H+��SO42-���ʴ�Ϊ��H+��SO42-��
��2��ԭ��Һ��һ��û��CO32-��NH4+���ʴ�Ϊ��CO32-��NH4+��
��3��ԭ��Һ�л����ܿ϶��Ƿ���ڵ���������Na+������������ӷ�������ɫ��Ӧ����ȡһ���ɾ��IJ�˿��պȡ��������Һ���ڻ������գ��۲��Ƿ���ֻ�ɫ���ʴ�Ϊ��ȡһ���ɾ��IJ�˿��պȡ��������Һ���ڻ������գ��۲��Ƿ���ֻ�ɫ��
�ʴ�Ϊ��Na+��ȡһ���ɾ��IJ�˿��պȡ��������Һ���ڻ������գ��۲��Ƿ���ֻ�ɫ��
������������Ҫ������dz��������Ӻ������ӵļ��飬��������������Ӧ�ǽ������Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
����þ���ܶȽ�С��þ�Ͻ��ǿ�ȸߡ���е���ܺã��������������ɻ����������Ҫ���ϣ��Ӷ���á��������������������ӻ�ѧԭ���;���Ч��Ƕȷ��������к�ˮ����ȡþ�ķ�����������ǣ�������
A����ˮ
| |||||||||||
B����ˮ
| |||||||||||
C����ˮ
| |||||||||||
D����ˮ
|
�����ʷ��������й����У�������ǣ�������
| A������ | B������ |
| C������ | D������Ũ���� |
������ͼ��ʾ��ԭ��أ�����˵����ȷ���ǣ�������
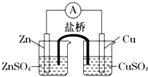

| A��п�ǵ�صĸ�����������ԭ��Ӧ |
| B�������е�������������ͭ��Һ��Ǩ�� |
| C��������п�缫ͨ������������ͭ�缫 |
| D��ͭ�缫�Ϸ����ĵ缫��Ӧ��2H++e-=H2�� |
���й����Ȼ�ѧ��Ӧ����������ȷ���ǣ�������
A�������ǵ�ȼ������2800 kJ?mol-1����
| ||
B��ȼ�ϵ���н��״�����ת��Ϊ�������Ȼ�ѧ����ʽ�ǣ�CH3OH��g��+
��CH3OH��g����ȼ����Ϊ192.9 kJ?mol-1 | ||
| C��H2��g����ȼ������285.8 kJ?mol-1����2H2O��g���T2H2��g��+O2��g����H=+571.6 kJ?mol-1 | ||
| D����֪H+��aq��+OH-��aq���TH2O��l����H=-57.3 kJ?mol-1����H2SO4��Ba��OH��2��Ӧ�ķ�Ӧ�ȡ�H=2����-57.3��kJ?mol-1 |
�������ӷ�Ӧ������д��ȷ���ǣ�������
| A��H2O+H2O?H3O++OH- |
| B��CO32-+2H2O?H2CO3+2OH- |
| C��Ca��OH��2+2H+?Ca2++2H2O |
| D��Al3++3H2O�TAl��OH��3��+3H+ |
��V L����KCl��BaCl2�Ļ����Һ�ֳ����ȷݣ�һ�ݼ��뺬a mol AgNO3����Һ��ǡ��ʹCl-��ȫ����ΪAgCl����һ�ݼ��뺬b mol Na2SO4����Һ��ǡ��ʹBa2+��ȫ����ΪBaSO4����ԭ�����Һ�м����ӵ�Ũ��Ϊ��������
A��
| ||
B��
| ||
C��
| ||
D��
|
���л�����Ӧ�����У�һ����������ԭ��Ӧ���ǣ�������
| A�����ֽⷴӦ | B���û���Ӧ |
| C���ֽⷴӦ | D�����Ϸ�Ӧ |
 �ס��ҡ����������졢��Ϊԭ��������������Ķ���������Ԫ�أ��ס�������ͬһ���壬���������촦��ͬһ���ڣ���Ԫ������ϼ�����ͼ۵Ĵ�����Ϊ0�����������������������ˮ����֮�䶼�ܷ�����Ӧ���û�ѧ����ش��������⣺
�ס��ҡ����������졢��Ϊԭ��������������Ķ���������Ԫ�أ��ס�������ͬһ���壬���������촦��ͬһ���ڣ���Ԫ������ϼ�����ͼ۵Ĵ�����Ϊ0�����������������������ˮ����֮�䶼�ܷ�����Ӧ���û�ѧ����ش��������⣺