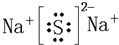��Ŀ����
�������ж�����Ԫ�����ʵ�������ݣ�
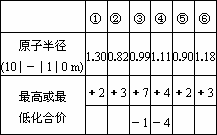
��ش��������⣺
(1)�ں͢��γɵĻ���������Ϊ________(����ӡ����ۡ�)�����
(2)Ԫ�آٵĽ����Ա�Ԫ�آ�Ҫ________(�ǿ��������)���Դ�ԭ�ӽṹ���������ԭ��________________��
(3)����Ȼ���У�Ԫ�آܵĴ�����̬Ϊ________����ҵ�ϴ��Ƹ�Ԫ�ص��ʵĻ�ѧ����ʽΪ________________��
(4)�ĵ�����ŨNaOH��Һ��Ӧ�����ӷ���ʽ��________________��
(5)ʵ��������Ԫ�آٵĵ��ʺ�ijδ֪��������M�����Ҫд���Ƚ����߽�����ǿ����һ��ʵ�鷽��________________��
�𰸣�

��ϰ��ϵ�д�
�����Ŀ