��Ŀ����
���Ȼ��ƹ�������1.00mol/L��NaCl��Һ95mL���ش���������
��1����������Ϊ��������ƽ����Ͳ��50mL�ձ��� mL����ƿ����Ҫ���ʵ�飬����Ҫ���ֲ�������Ϊ�� �� ��
��2����д����ʵ���ʵ�鲽�裺
�ټ��㣬�ڳ��� gNaCl�����ܽ⣬����Һ���� ���� ����ҡ�ȣ�
��3���Է������в�������������Һ��Ũ���к�Ӱ�죮����ƫ�͡�ƫ�ߡ���Ӱ����գ���
������ƽ�����Ȼ��ƺ�������ײ�����ߣ�������Һ��Ũ�ȣ� ��
��Ϊ���ٹ����ܽ⣬���ձ���Һ�����Ͻ��裮��δ����20��ʱ���ͽ���Һת��������ƿ���ݣ�������Һ��Ũ�ȣ� ��
�۶��ݺ��ӿ̶�����������Һ��Ũ�ȣ� ��
�ܶ��ݺӸǡ���ת��ҡ�Ⱥ���Һ����ڿ̶��ߣ��ֲ�������ˮ���̶ȣ���������Һ��Ũ�ȣ� ��
����һͬѧȷ������Һ����ȡ��25mLϡ�ͳ�l00mL��ϡ�ͺ�NaCl�����ʵ���Ũ�� Ϊ ��
��1����������Ϊ��������ƽ����Ͳ��50mL�ձ���
��2����д����ʵ���ʵ�鲽�裺
�ټ��㣬�ڳ���
��3���Է������в�������������Һ��Ũ���к�Ӱ�죮����ƫ�͡�ƫ�ߡ���Ӱ����գ���
������ƽ�����Ȼ��ƺ�������ײ�����ߣ�������Һ��Ũ�ȣ�
��Ϊ���ٹ����ܽ⣬���ձ���Һ�����Ͻ��裮��δ����20��ʱ���ͽ���Һת��������ƿ���ݣ�������Һ��Ũ�ȣ�
�۶��ݺ��ӿ̶�����������Һ��Ũ�ȣ�
�ܶ��ݺӸǡ���ת��ҡ�Ⱥ���Һ����ڿ̶��ߣ��ֲ�������ˮ���̶ȣ���������Һ��Ũ�ȣ�
����һͬѧȷ������Һ����ȡ��25mLϡ�ͳ�l00mL��ϡ�ͺ�NaCl�����ʵ���Ũ�� Ϊ
���㣺��Һ������
ר�⣺ʵ����
��������1����������һ�����ʵ���Ũ����Һ�IJ�������ѡ������������������Һ�����ѡ����ʵ�����ƿ��
��2������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������n=C��V��m=n��M������Ҫ���Ȼ��Ƶ�������
��3������C=
�����������ܹ�ʹnƫС����ʹVƫ��IJ�������ʹ��Һ��Ũ��ƫС����֮����Һ��Ũ��ƫ��
������ϡ��ǰ����Һ�к����ʵ����ʵ����������ϡ�ͺ���Һ��Ũ�ȣ�
��2������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������n=C��V��m=n��M������Ҫ���Ȼ��Ƶ�������
��3������C=
| n |
| V |
������ϡ��ǰ����Һ�к����ʵ����ʵ����������ϡ�ͺ���Һ��Ũ�ȣ�
���
�⣺��1��ʵ����û��95mL����ƿ���ϸ�ѡ��100mL����ƿ��ʵ�������Ƶ���ҺΪ100mL 1.00mol/L���Ȼ�����Һ���������Ʋ����֪�����Ƹ���Һ��Ҫ�������У�������ƽ����Ͳ��50mL�ձ���100mL����ƿ������������ͷ�ιܵȣ���ȱ�ٵIJ�������Ϊ����ͷ�ιܺͲ�������
�ʴ�Ϊ��100����ͷ�ιܣ���������
��2�����Ƶ���ҺΪ100mL 1.00mol/L���Ȼ�����Һ����Ҫ�Ȼ��Ƶ�����Ϊ��1.00mol/L��0.1L=0.1mol��������m=58.5g/mol��0.1mol��5.9g�����Ƹ���Һ�IJ���Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ�
�ʴ�Ϊ��5.9��ϴ�ӣ����ݣ�
��3������C=
�����������ܹ�ʹnƫС����ʹVƫ��IJ�������ʹ��Һ��Ũ��ƫС����֮����Һ��Ũ��ƫ��
������ƽ�����Ȼ��ƺ�������ײ�����ߣ����ȡ���Ȼ��Ƶ�����ƫ�����ʵ���nƫ����ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��Ϊ���ٹ����ܽ⣬���ձ���Һ�����Ͻ��裮��δ����20��ʱ���ͽ���Һת��������ƿ���ݣ���Һ��ȴ��Һ����ڿ̶��ߣ���Һ�����VƫС����Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
�۶��ݺ��ӿ̶�����������Һ��Ũ�ȣ�������Һ�����VƫС����Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
�ܶ��ݺӸǡ���ת��ҡ�Ⱥ���Һ����ڿ̶��ߣ��ֲ�������ˮ���̶ȣ�������Һ�����Vƫ����Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��ϡ��ǰ����Һ�к����ʵ����ʵ������䣬��ϡ�ͺ���Һ�����ʵ���Ũ��ΪC��1.00mol/L��25mL=100ml��
C�����C=0.25mol/L��
�ʴ�Ϊ��0.25mol/L��
�ʴ�Ϊ��100����ͷ�ιܣ���������
��2�����Ƶ���ҺΪ100mL 1.00mol/L���Ȼ�����Һ����Ҫ�Ȼ��Ƶ�����Ϊ��1.00mol/L��0.1L=0.1mol��������m=58.5g/mol��0.1mol��5.9g�����Ƹ���Һ�IJ���Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ�
�ʴ�Ϊ��5.9��ϴ�ӣ����ݣ�
��3������C=
| n |
| V |
������ƽ�����Ȼ��ƺ�������ײ�����ߣ����ȡ���Ȼ��Ƶ�����ƫ�����ʵ���nƫ����ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��Ϊ���ٹ����ܽ⣬���ձ���Һ�����Ͻ��裮��δ����20��ʱ���ͽ���Һת��������ƿ���ݣ���Һ��ȴ��Һ����ڿ̶��ߣ���Һ�����VƫС����Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
�۶��ݺ��ӿ̶�����������Һ��Ũ�ȣ�������Һ�����VƫС����Һ��Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
�ܶ��ݺӸǡ���ת��ҡ�Ⱥ���Һ����ڿ̶��ߣ��ֲ�������ˮ���̶ȣ�������Һ�����Vƫ����Һ��Ũ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��ϡ��ǰ����Һ�к����ʵ����ʵ������䣬��ϡ�ͺ���Һ�����ʵ���Ũ��ΪC��1.00mol/L��25mL=100ml��
C�����C=0.25mol/L��
�ʴ�Ϊ��0.25mol/L��
���������⿼��������һ�����ʵ���Ũ�ȵ���Һ����Ŀ�Ѷ��еȣ���������һ�����ʵ���Ũ�ȵ���Һ���輰ѡ�������ķ�������ȷ�������ķ����뼼�ɣ��ǽ���ؼ�������������ѧ�����Ӧ����ѧ֪ʶ��������

��ϰ��ϵ�д�
 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�
�����Ŀ
 ��ͼΪԭ���װ��ʾ��ͼ��
��ͼΪԭ���װ��ʾ��ͼ��
 �к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�飮ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L��������Һ50mL������ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺
�к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�飮ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L��������Һ50mL������ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺ ��9��CH2=CH-CH3��8��
��9��CH2=CH-CH3��8�� ��10��
��10�� ��11��2��2-��������
��11��2��2-��������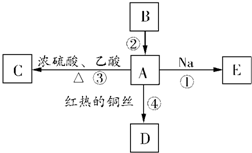 A�ǻ�ѧʵ������������л����������ˮ����������ζ��B�IJ����ɺ���һ������ʯ�ͻ�����չ��ˮƽ���й����ʵ�ת����ϵ��ͼ��ʾ��
A�ǻ�ѧʵ������������л����������ˮ����������ζ��B�IJ����ɺ���һ������ʯ�ͻ�����չ��ˮƽ���й����ʵ�ת����ϵ��ͼ��ʾ��