��Ŀ����
ij��ѧʽΪAB�����Ӿ��壬��֪5r��A2+��=4r��B2-�����������Ӿ������������Ӱ뾶�ȹ�ϵ��ͼ��ʾ�������ⶨ���ܶ�Ϊ��g?cm-3����Ħ������ΪMg?mol-1���������йظþ����˵����ȷ���ǣ�������
| r+/r- | ��λ�� | ʵ�� |
| 0.225��0.414 | 4 | ZnS |
| 0.414��0.732 | 6 | NaCl |
| 0.732��1.0 | 8 | CsCl |
| ��1.0 | 12 | CsF |
| A���þ���ṹ��ZnS�������� | |||||
| B���þ���ÿ����������2��A2+��B2- | |||||
C���þ��徧���ı߳�
| |||||
| D��A2+���ӽ��ڵ�B2-���ɵĿռ�ṹΪ������ |
���㣺�����ļ���
ר�⣺
������A������5r��A2+��=4r��B2-�����ж��������������Ӱ뾶֮�ȣ��ж������Ƶľ���ṹ��
B�����ڸþ�����CsCl�������ƣ���������λ�����ж��侧��ṹ���Ӷ���þ�������������������
C������V=
��������߳���
D��A2+����λ�ھ������ģ�B2-����λ�ھ�����8�����㣬���ݿռ����������ж���ռ�ṹ��
B�����ڸþ�����CsCl�������ƣ���������λ�����ж��侧��ṹ���Ӷ���þ�������������������
C������V=
| m |
| �� |
D��A2+����λ�ھ������ģ�B2-����λ�ھ�����8�����㣬���ݿռ����������ж���ռ�ṹ��
���
�⣺A������5r��A2+��=4r��B2-������r+/r-=0.8�����ݱ������ݿ�֪���þ�����������������Ӱ뾶֮����CsCl���ƣ���A����
B�����ڸþ�����CsCl�������ƣ�����λ��Ϊ8����������Ϊ����������������Ϊһ�������壬���������ӷֱ�λ�ھ����Ķ�������ģ���������Ϊ1����B����
C��һ�������к���1���������ӣ���һ����������Ϊ��
���������V=
������Ϊ�����壬��߳�Ϊ��
����C����
D��A2+����λ�ھ������ģ�B2-����λ�ھ�����8�����㣬�ռ乹��Ϊ�����壬��D��ȷ��
��ѡD��
B�����ڸþ�����CsCl�������ƣ�����λ��Ϊ8����������Ϊ����������������Ϊһ�������壬���������ӷֱ�λ�ھ����Ķ�������ģ���������Ϊ1����B����
C��һ�������к���1���������ӣ���һ����������Ϊ��
| M |
| NA |
| M |
| NA?�� |
| 3 |
| ||
D��A2+����λ�ھ������ģ�B2-����λ�ھ�����8�����㣬�ռ乹��Ϊ�����壬��D��ȷ��
��ѡD��
���������⿼�龧���ṹ����һ�����Ѷȣ�Ӧ��Ϥ���Ӿ����и��־������ͣ�����������Ӧ�þ���������ܶȺ�Ħ������֮����ת����

��ϰ��ϵ�д�
�����Ŀ
�����и������ʰ����ʡ�������ᡢ��η���˳�����У�������ȷ���ǣ�������
| A�������ɱ������ᡢ�ռʳ�� |
| B����ơ��������ᡢ�ռʳ�� |
| C�������������������ᡢ�������� |
| D��ͭ������ͭ�����ᡢʯ��ˮ���Ȼ�ͭ |
�����з�Ӧ�У�HCl ��������ã�������
��NaOH+HCl=NaCl+H2O
��Zn+2HCl=ZnCl2+H2��
��MnO2+4HCl��Ũ��
MnCl2+2H2O+Cl2��
��CuO+2HCl=CuCl2+H2O��
��NaOH+HCl=NaCl+H2O
��Zn+2HCl=ZnCl2+H2��
��MnO2+4HCl��Ũ��
| ||
��CuO+2HCl=CuCl2+H2O��
| A���٢ܱ��ֳ����� |
| B���٢ڱ��ֳ������� |
| C���ۼȱ��ֳ��������ֱ��ֳ����� |
| D���ܱۢ��ֳ���ԭ�� |
 ij����С����ʵ����������̽��CO��Fe2O3��Ӧ�IJ��������ʵ�������ͼ��ʾ��
ij����С����ʵ����������̽��CO��Fe2O3��Ӧ�IJ��������ʵ�������ͼ��ʾ��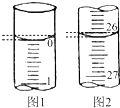 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ���̪��ָʾ��������д���пհף� �-������ͭ��������Ļ���������Cu4O��PO4��2����ͨ�����з�Ӧ�Ʊ���2Na3PO4+4CuSO4+2NH3?H2O=Cu4O��PO4��2��+3Na2SO4+��NH4��2SO4+H2O
�-������ͭ��������Ļ���������Cu4O��PO4��2����ͨ�����з�Ӧ�Ʊ���2Na3PO4+4CuSO4+2NH3?H2O=Cu4O��PO4��2��+3Na2SO4+��NH4��2SO4+H2O ijѧ������ѧϰ�С����Խ̲���ͭ��Ũ���ᷴӦ��������о����ܹ���ͭ��Ӧ����������Ũ���Ƕ��٣��������⣬����������·�������ʵ�飺
ijѧ������ѧϰ�С����Խ̲���ͭ��Ũ���ᷴӦ��������о����ܹ���ͭ��Ӧ����������Ũ���Ƕ��٣��������⣬����������·�������ʵ�飺