��Ŀ����
2�������꿪���ļ״�ȼ�ϵ���Dz��ò����缫����������е����ӽ���Ĥֻ�������Ӻ�ˮ����ͨ�����乤��ԭ����ʾ��ͼ��ͼ��
��ش��������⣺
��1��Pt��a���缫�ǵ�صĸ������缫��ӦΪCH3OH+H2O-6e-�TCO2��+6H+��Pt��b���缫������ԭ��Ӧ�����������ԭ�������缫��ӦΪO2+4H++4e-�T2H2O��
��2����ص��ܷ�Ӧ����ʽΪCH4+2O2�TCO2+2H2O��
��3������õ�ع���ʱ��·��ͨ��6mol���ӣ������ĵ�CH3OH��1 mol��
���� ��ȼ�ϵ���У��������Һ�����ԣ���������������������Pt��a��������ȼ��ʧ���ӷ���������Ӧ��Pt��a���缫��ӦʽΪCH4-8e-+2H2O�TCO2+8H+��Pt��b���缫Ϊ�������缫��ӦʽΪO2+4e-+4H+�T2H2O���ڵ�ʧ������ȵ������£��������缫��Ӧʽ��Ӽ��õ�ط�Ӧʽ����ϼ���͵���֮��Ĺ�ϵʽ���㣮
��� �⣺��1��ȼ�ϵ���У��������Һ�����ԣ���������������������Pt��a��������ȼ��ʧ���ӷ���������Ӧ��Pt��a���缫��ӦʽΪCH4-8e-+2H2O�TCO2+8H+��Pt��b���缫Ϊ������������ԭ��Ӧ���缫��ӦʽΪO2+4e-+4H+�T2H2O���ʴ�Ϊ������CH3OH+H2O-6e-�TCO2��+6H+����ԭ��O2+4H++4e-�T2H2O��
��2���������缫��Ӧʽ��Ӽ��õ�ط�ӦʽΪCH4+2O2�TCO2+2H2O���ʴ�Ϊ��CH4+2O2�TCO2+2H2O��
��3���ɵ缫����ʽCH3OH+H2O-6e-�TCO2+6H+��֪������õ�ع���ʱ��·��ͨ��6mol���ӣ������ĵ�CH3OH��1mol���ʴ�Ϊ��1��
���� ���⿼��ԭ���ԭ�������ؿ���缫��Ӧʽ��д����ϵ������Һ��д�缫��Ӧʽ��ע�⣺��д�����²����������������ӣ����������²������������ӣ���Ŀ�Ѷ��еȣ�

| A�� | �ױ��ķ���ʽΪ��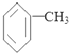 | |
| B�� | �ױ�����������ԭ�Ӷ�����ͬһƽ�� | |
| C�� | �ױ���һ��ȡ������5��ͬ���칹�壬���ǵ��۵㡢�е������ͬ | |
| D�� | �ױ��ͱ���Ϊͬϵ�� |
| A�� | NaCl | B�� | NaOH | C�� | HCl | D�� | Cl2 |
| A�� | ����Ũ���ᷴӦ | B�� | �Ҵ���HBr��Һ������������ | ||
| C�� | �Ҵ���Ũ���Ṳ����170�� | D�� | ��֬��ǿ��ˮ��Һ��Ӧ |
| A�� | 1 mol C4H10�����й��ۼ�����Ϊ13NA | |
| B�� | 1L 1mol•L-1 �����У�����HCl������ΪNA | |
| C�� | 18g D2O �к��е��������͵�������Ϊ10NA | |
| D�� | 1molCl2��Fe ��Ӧת�Ƶĵ�����ĿΪ3NA |
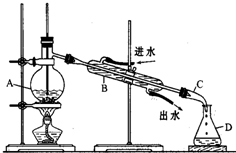 ijͬѧ���������װ�ý���ʯ�������ʵ�飬�ش������й����⣺
ijͬѧ���������װ�ý���ʯ�������ʵ�飬�ش������й����⣺ Fe��OH��3�����壩+3H+�����ɵ�����Fe��OH��3�����������ˮ�е��������ʣ��ﵽ��ˮ��Ŀ�ģ�
Fe��OH��3�����壩+3H+�����ɵ�����Fe��OH��3�����������ˮ�е��������ʣ��ﵽ��ˮ��Ŀ�ģ�