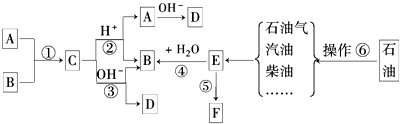
��Ŀ����
ij��Һ�п��ܺ�������6�������е�ij���֣�Cl-��SO42-��NH4+��CO32-��K+��Na+��Ϊȷ����Һ��ɽ�������ʵ�飺
��200mL������Һ����������BaCl2��Һ����Ӧ�������ˡ�ϴ�ӡ�����ó���4.30g��������м�����������ᣬ��2.33g�������ܣ�
����ٵ���Һ�м���������NaOH��Һ�����ȣ������ܴ�ʹʪ���ɫʯ����ֽ����������1.12L���ѻ���ɱ�״�����ٶ�����������ȫ���ݳ�����
��1����Һһ�����ڵ������� �����ܴ��ڵ������� ��
��2��ԭ��Һ��c��CO32-��Ϊ ��c��NH4+�� c��SO42-�� �����������=������
��3���������6�����Ӷ����ڣ���c��Cl-�� c��SO42-�� �����������=������
��200mL������Һ����������BaCl2��Һ����Ӧ�������ˡ�ϴ�ӡ�����ó���4.30g��������м�����������ᣬ��2.33g�������ܣ�
����ٵ���Һ�м���������NaOH��Һ�����ȣ������ܴ�ʹʪ���ɫʯ����ֽ����������1.12L���ѻ���ɱ�״�����ٶ�����������ȫ���ݳ�����
��1����Һһ�����ڵ�������
��2��ԭ��Һ��c��CO32-��Ϊ
��3���������6�����Ӷ����ڣ���c��Cl-��
���㣺���������ӵļ���,���������ӵļ���
ר�⣺���ʼ��������
��������ȡ��������Һ����BaCl2��Һ�а�ɫ�������ɣ��ټ�������������������ܽ⣬˵����ɫ����ΪBaCO3��BaSO4������Һ�к���CO32-��SO42-��
����ٵ���Һ�м���������NaOH��Һ�����ȣ�������ʹʪ���ɫʯ����ֽ���������壬˵����Һ����NH4+��
����ٵ���Һ�м���������NaOH��Һ�����ȣ�������ʹʪ���ɫʯ����ֽ���������壬˵����Һ����NH4+��
���
�⣺��ȡ��������Һ����BaCl2��Һ�а�ɫ�������ɣ��ټ�������������������ܽ⣬�����������ɣ�˵����ɫ����ΪBaCO3��BaSO4������һ����4.3g������Һ�к���CO32-��SO42-��������м�����������ᣬ��2.33g�������ܣ������ᱵ��������2.33g��������������ӵ����ʵ�����
=0.01mol������̼�ᱵ��������4.3g-2.33g=1.97g��̼������ӵ����ʵ�����
=0.01mol��
����ٵ���Һ�м���������NaOH��Һ�����ȣ�������ʹʪ���ɫʯ����ֽ�����������ǰ��������ʵ�����
=0.05mol��˵����Һ����NH4+�����ʵ�����0.05mol���ۺ����Ϸ�����
��1����Һһ�����ڵ������У�CO32-��SO42-��NH4+�����ܴ��ڵ������У�Cl-��K+��Na+���ʴ�Ϊ��CO32-��SO42-��NH4+��Cl-��K+��Na+��
��2�����ݼ���ó�c��CO32-��=
=0.05mol/L�������Ϊ0.01mol��笠�Ϊ0.05mol����c��NH4+������SO42-�����ʴ�Ϊ��0.05mol/L��c��NH4+������SO42-����
��3��������Һ������ԭ���趼���ڣ���ôn��+��=n��-������0.05+n��Na+��+n��K+��=2��0.01+2��0.01+n��Cl-�����ݴ˵ó�n��Cl-��=n��Na+��+n��K+��+0.01��0.01���ʴ�Ϊ������
| 2.33g |
| 233g/mol |
| 1.97g |
| 197g/mol |
����ٵ���Һ�м���������NaOH��Һ�����ȣ�������ʹʪ���ɫʯ����ֽ�����������ǰ��������ʵ�����
| 1.12L |
| 22.4L/mol |
��1����Һһ�����ڵ������У�CO32-��SO42-��NH4+�����ܴ��ڵ������У�Cl-��K+��Na+���ʴ�Ϊ��CO32-��SO42-��NH4+��Cl-��K+��Na+��
��2�����ݼ���ó�c��CO32-��=
| 0.01mol |
| 0.2L |
��3��������Һ������ԭ���趼���ڣ���ôn��+��=n��-������0.05+n��Na+��+n��K+��=2��0.01+2��0.01+n��Cl-�����ݴ˵ó�n��Cl-��=n��Na+��+n��K+��+0.01��0.01���ʴ�Ϊ������
������������Ҫ������dz��������Ӽ�����ۺ��⣬��һ���Ѷȣ�ǣ�����ӵĶ����ж�����㣮

��ϰ��ϵ�д�
 �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д�
�����Ŀ
���л�ѧ��ʵ������۶���ȷ���ǣ�������
| A������������ò�Ҫʢ�����ԡ�����Խ�ǿ��Һ��ʳ���ΪAl��Al2O3�ȿ������ᷴӦ���ֿ�����Ӧ |
| B����SO2ͨ�뺬HClO����Һ�У�����H2SO4��˵��HClO���Ա�H2SO4 ǿ |
| C��FeCl3��Һ���Ը�ʴ��·���ϵ�Cu��˵��Fe�Ľ�����Դ���Cu |
| D��������ͭм��ϡ�������ã���Ӧֹͣ���ټ���1mol/Lϡ���ᣬͭм�����ܽ�����Ϊͭ��ֱ����1mol/Lϡ���ᷴӦ |
�����й�ʵ��ԭ���������ͽ��۶���ȷ���ǣ�������
| A������ҺX���ȵμ�ϡ���ᣬ�ٵμ�Ba��NO3��2��Һ�����ְ�ɫ����˵����ҺX��һ������SO42- |
| B��ȡ������ҺX�������м�������������ˮ���ټӼ���KSCN��Һ����Һ��죬˵��X��Һ��һ������Fe2+ |
| C����������Ũ��Ϊ2mol?L-1��NaCl��Һ���ɽ�58.5g NaCl���뵽ʢ��500mLˮ���ձ��У����衢�ܽ� |
| D����ij���������ɫ��Ӧʵ�飬����ʻ�ɫ��˵��������Ϊһ������ |
���и������������ǣ�������
| A���þƾ���ȡ��ˮ�е��嵥�ʵIJ�����ѡ�÷�Һ©���������÷�Һ |
| B����Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| C����ȡ����Һǰ��Է�Һ©����© |
| D��Ϊ��֤��Һ©���ڵ�Һ��˳���������轫������������� |
��һ�λ�ѧʵ����������У�ij��ͬѧ�����¼��ֲ�����Ӧ����ʩ�����в���ȷ���ǣ�������
A�� �ƾ�ʧ����ʪĨ���˸� |
B�� ��NaCl������Ͳ��������Һ |
C�� ��ĥ����ζ����ӷ��Թ��� |
D�� ������Һ��������KNO3���� |