��Ŀ����
���ʽṹ
��������Ԫ�صĵ��ʼ��������ڹ�ũҵ������Ӧ�ù㷺��
��1����������Ԫ���У���̬ԭ�ӵ������ֻ��1�����ӵ�Ԫ���У��ֱ�д���������壬����Ԫ�ظ�һ��Ԫ�صĺ�������Ų�ʽ
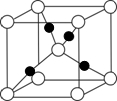
��2����ˮ����ͭ�ʰ�ɫ����ˮ���γɵ���������ɫ����ѧ��ͨ��X���߲ⶨ�������Ľṹ����ṹ������ͼ��ʾ��
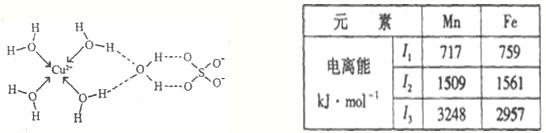
�ٵ��������г����ۼ�����λ���������ڵ�������������
��SO42-���ӳ���������ṹ��������ԭ�ӵ��ӻ����������
��ͭ�����γɵ�������λ���ӵ����ӷ���ʽΪ
��3��Mn��Fe��Ϊ�������ڹ���Ԫ�أ���Ԫ�صIJ��ֵ���������������ݱ��ж�Mn2+��Fe2+��ʧȥ-�����ӵ����ף�����ԭ�ӽṹ�ĽǶȼ�����ԭ��
��������Ԫ�صĵ��ʼ��������ڹ�ũҵ������Ӧ�ù㷺��
��1����������Ԫ���У���̬ԭ�ӵ������ֻ��1�����ӵ�Ԫ���У��ֱ�д���������壬����Ԫ�ظ�һ��Ԫ�صĺ�������Ų�ʽ
K[Ar]4s1��Cu[Ar]3d104s1
K[Ar]4s1��Cu[Ar]3d104s1
����2����ˮ����ͭ�ʰ�ɫ����ˮ���γɵ���������ɫ����ѧ��ͨ��X���߲ⶨ�������Ľṹ����ṹ������ͼ��ʾ��

�ٵ��������г����ۼ�����λ���������ڵ�������������
������Ӽ�
������Ӽ�
����SO42-���ӳ���������ṹ��������ԭ�ӵ��ӻ����������
sp3
sp3
����ͭ�����γɵ�������λ���ӵ����ӷ���ʽΪ
Cu2++4H2O=[Cu��H2O��4]2+
Cu2++4H2O=[Cu��H2O��4]2+
����3��Mn��Fe��Ϊ�������ڹ���Ԫ�أ���Ԫ�صIJ��ֵ���������������ݱ��ж�Mn2+��Fe2+��ʧȥ-�����ӵ����ף�����ԭ�ӽṹ�ĽǶȼ�����ԭ��
Mn2+��ʧȥ���ӱ�Fe2+���ѣ�ԭ����Mn2+��3d5�����ڽ��ȶ��İ�����ṹ��
Mn2+��ʧȥ���ӱ�Fe2+���ѣ�ԭ����Mn2+��3d5�����ڽ��ȶ��İ�����ṹ��
����������1�����ݺ�������Ų�������д����������Ԫ���У���̬ԭ�ӵ������ֻ��1�����ӵ�Ԫ���У�������K������Ԫ��ΪCu�ȣ�
��2������ͼ��֪H��O֮����������ͭ�����������֮��������Ӽ���
����ͼ��֪S��4���ļ���SO42-���ӳ���������ṹ���ɴ��ж�S���ӻ���ʽ��
��ͭ������ˮ����ͨ����λ���γ�ˮ��ͭ����[Cu��H2O��4]2+��
��3��������Խ��Խ������ʧȥ���ӣ����ݵ��������ܿ�֪Mn2+��ʧȥ���ӱ�Fe2+���ѣ����Ӵ��ڰ�����ȫ����ȫ���Ǹ��ȶ���
��2������ͼ��֪H��O֮����������ͭ�����������֮��������Ӽ���
����ͼ��֪S��4���ļ���SO42-���ӳ���������ṹ���ɴ��ж�S���ӻ���ʽ��
��ͭ������ˮ����ͨ����λ���γ�ˮ��ͭ����[Cu��H2O��4]2+��
��3��������Խ��Խ������ʧȥ���ӣ����ݵ��������ܿ�֪Mn2+��ʧȥ���ӱ�Fe2+���ѣ����Ӵ��ڰ�����ȫ����ȫ���Ǹ��ȶ���
����⣺��1����������Ԫ���У���̬ԭ�ӵ������ֻ��1�����ӵ�Ԫ���У�������K�����������Ų�ʽΪ[Ar]4s1��
����Ԫ����Cu�ȣ�Cu��������Ų�ʽΪ[Ar]3d104s1��
�ʴ�Ϊ��K[Ar]4s1�� Cu[Ar]3d104s1
��2��������ͼ��֪H��O֮����������ͭ�����������֮��������Ӽ���
�ʴ�Ϊ����� ���Ӽ�
����ͼ��֪S��4���ļ���SO42-��S�Ļ��ϼ�Ϊ+6�ۣ���������ȫ���ɼ����¶Ե��ӣ�����S��ȡsp3�ӻ���ʽ
�ʴ�Ϊ��sp3
��ͭ������ˮ����ͨ����λ���γ�ˮ��ͭ����[Cu��H2O��4]2+�����ӷ���ʽΪCu2++4H2O=[Cu��H2O��4]2+��
�ʴ�Ϊ��Cu2++4H2O=[Cu��H2O��4]2+��
��3��Mn��I3=3248 kJ?mo1-1����Fe��I3=2957 kJ?mo1-1������Mn2+��ʧȥ���ӱ�Fe2+���ѣ�ԭ����Mn2+��3d5�����ڽ��ȶ��İ�����ṹ��
�ʴ�Ϊ��Mn2+��ʧȥ���ӱ�Fe2+���ѣ�ԭ����Mn2+��3d5�����ڽ��ȶ��İ�����ṹ��
����Ԫ����Cu�ȣ�Cu��������Ų�ʽΪ[Ar]3d104s1��
�ʴ�Ϊ��K[Ar]4s1�� Cu[Ar]3d104s1
��2��������ͼ��֪H��O֮����������ͭ�����������֮��������Ӽ���
�ʴ�Ϊ����� ���Ӽ�
����ͼ��֪S��4���ļ���SO42-��S�Ļ��ϼ�Ϊ+6�ۣ���������ȫ���ɼ����¶Ե��ӣ�����S��ȡsp3�ӻ���ʽ
�ʴ�Ϊ��sp3
��ͭ������ˮ����ͨ����λ���γ�ˮ��ͭ����[Cu��H2O��4]2+�����ӷ���ʽΪCu2++4H2O=[Cu��H2O��4]2+��
�ʴ�Ϊ��Cu2++4H2O=[Cu��H2O��4]2+��
��3��Mn��I3=3248 kJ?mo1-1����Fe��I3=2957 kJ?mo1-1������Mn2+��ʧȥ���ӱ�Fe2+���ѣ�ԭ����Mn2+��3d5�����ڽ��ȶ��İ�����ṹ��
�ʴ�Ϊ��Mn2+��ʧȥ���ӱ�Fe2+���ѣ�ԭ����Mn2+��3d5�����ڽ��ȶ��İ�����ṹ��
�����������������Ų�ʽ����ѧ����ԭ�ӵĽṹ�����ʹ�ϵ�Լ�ѧ����ͼ����ȡ��Ϣ�������Ѷ��еȣ�ּ�ڿ���ѧ����֪ʶ�����������ã�

��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
�����Ŀ
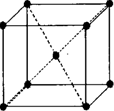 ��2011?�����ģ��[ѡ�����ʽṹ������]��֪A��B��C��D��E����Ԫ�صĺ˵������������EΪ��������Ԫ���⣬����Ƕ�����Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AԪ���������������ڲ��������2����BԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��EԪ�ص�+3�����ӵ�3d�ܼ�Ϊ�����״̬��������ʱ��ABCDE��Ӧ��Ԫ�ط��ű�ʾ��
��2011?�����ģ��[ѡ�����ʽṹ������]��֪A��B��C��D��E����Ԫ�صĺ˵������������EΪ��������Ԫ���⣬����Ƕ�����Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AԪ���������������ڲ��������2����BԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��EԪ�ص�+3�����ӵ�3d�ܼ�Ϊ�����״̬��������ʱ��ABCDE��Ӧ��Ԫ�ط��ű�ʾ��



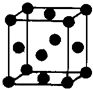 ��2012?�人ģ�⣩[��ѧһѡ�� 3�����ʽṹ������]��U��V��W��X��Y��Z ����ǰ������Ԫ�أ�ԭ���������������������Ϣ���±���
��2012?�人ģ�⣩[��ѧһѡ�� 3�����ʽṹ������]��U��V��W��X��Y��Z ����ǰ������Ԫ�أ�ԭ���������������������Ϣ���±���