��Ŀ����
19�����Ӽ������������ڰ뵼�幤ҵ�͵����մɵ�������һ�ִ��Ⱥܸߵ�������乤ҵ��ȡ������ͼ1��
��1������A�ijɷֳ�Co�������κ�Fe��OH��3���Si ���ѧʽ����
��2������Co3��PO4��2��Ŀ���ǵ�����ҺpH����֤Co2+����������ʹ������ȫ��������
��3��Co��ϡ���ᷴӦ����Co2+�����ӷ���ʽΪ3Co+8H++2NO3-�T3Co2++4H2O+2NO����
��4������B��������ϴ�ӡ�����������գ������ط���ͼ��ͼ2��
д��C�����ʵĻ�ѧʽ��Co3O4��
��5�������Ʊ������в��ò�������Ϊ�����������������ɱ��ϸߣ�ij����С���о����������Һ����Ҫ�ɷ�ΪCoSO4��Fe2��SO4��3��Al2��SO4��3����������̼泥�NH4HCO3������������������������ɴ��Ʊ������ܣ����������Ϣ����������ʵ�鲽�裮
��֪��a�������������������������pH���±�����ʼ����pH����������1.0mol/L���㣩
| ��ʼ������pH | ��ȫ������pH | |
| Fe3+ | 1.1 | 3.2 |
| Al3+ | 3.0 | 5.0 |
| Co2+ | 7.2 | 9.2 |
���������Һ�Ʊ������ܵ�ʵ�鲽������Ϊ��
�����������Һ�еμ�NaOH��Һ��������ҺpH��5.0��7.2����ʹFe3+��Al3+ ������ȫ��
�ڹ��ˣ�������һ��Ũ�ȵ�̼���Һ���������뵽��Һ�У����裬����pH��7.0���ң�ʹCo2+������ȫ�����ˣ�
�ܽ�����ˮ������70�����ϣ�ϴ��CoCO3����3�Σ������400�決��3h���ò�Ʒ�����ܣ�
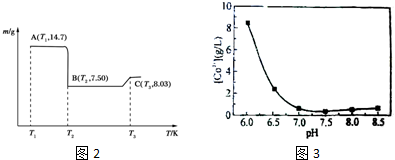
���� ��ҵ��Co����ϡ����������ܣ��õ�����A����CoSO4��Fe��OH��3��Si����ҺB����Co2+��������������CoC2O4����ϴ�ӡ�������յõ������ܣ�
��1����������Ӧ��
��2����������ˮ�⣬����Co3��PO4��2�ɵ�����ҺpH��ʹ������ȫ��������
��3��Co�����ᷢ��������ԭ��Ӧ��
��4��AΪCoC2O4��n��CoC2O4��=$\frac{14.7g}{147g/mol}$=0.1mol��m��Co��=0.1mol��59g/mol=5.9g��C�������������Ϊ8.03g����n��O��=$\frac{8.03g-5.9g}{16g/mol}$=1.33���Դ˼��㣻
��5���ɱ������ݿ�֪��Ӧ�ȼ����������Ƴ�ȥFe3+��Al3+���ɵ���pH5.0��7.2֮�䣬Ȼ����ˣ�����Һ�м���һ��Ũ�ȵ�̼���Һ������pH��7.0���ң�ʹCo2+������ȫ�����ˣ�������ˮ������70�����ϣ�ϴ��CoCO3����3�Σ����������400�決��3h���ò�Ʒ�����ܣ�
��� �⣺��ҵ��Co����ϡ����������ܣ��õ�����A����CoSO4��Fe��OH��3��Si����ҺB����Co2+��������������CoC2O4����ϴ�ӡ�������յõ������ܣ�
��1����������Ӧ������A����CoSO4��Fe��OH��3��Si��
�ʴ�Ϊ��Si��
��2����������ˮ�⣬����Co3��PO4��2�ɵ�����ҺpH����֤Co2+����������ʹ������ȫ��������
�ʴ�Ϊ��������ҺpH����֤Co2+����������ʹ������ȫ��������
��3��Co�����ᷢ��������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ3Co+8H++2NO3-�T3Co2++4H2O+2NO����
�ʴ�Ϊ��3Co+8H++2NO3-�T3Co2++4H2O+2NO����
��4��AΪCoC2O4��n��CoC2O4��=$\frac{14.7g}{147g/mol}$=0.1mol��m��Co��=0.1mol��59g/mol=5.9g��C�������������Ϊ8.03g����n��O��=$\frac{8.03g-5.9g}{16g/mol}$=1.33����ѧʽΪCo3O4��
�ʴ�Ϊ��Co3O4��
��5���ɱ������ݿ�֪��Ӧ�ȼ����������Ƴ�ȥFe3+��Al3+���ɵ���pH5.0��7.2֮�䣬Ȼ����ˣ�����Һ�м���һ��Ũ�ȵ�̼���Һ������pH��7.0���ң�ʹCo2+������ȫ�����ˣ�������ˮ������70�����ϣ�ϴ��CoCO3����3�Σ����������400�決��3h���ò�Ʒ�����ܣ�
�ʴ�Ϊ����pH��5.0��7.2����Fe3+��Al3+���ۿ���pH��7.0���ң�ʹCo2+������ȫ�����ˣ�
���� ���⿼���������ᴿ�ͷ���Ĺ�ҵ�Ʊ����̷����жϣ�Ϊ�߿��������ͣ�������ѧ���ķ���������ʵ�������ͼ��������Ŀ��飬����ʵ����̵ķ���Ӧ�ã���Ҫ���������ʵ�����Ӧ�ã���Ŀ�ѶȽϴ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д��������̿��м������ϡH2SO4�����ˣ�
������Һ�м������MnO2�����ˣ�
������ҺpH=a�����ˣ�
����Ũ�����ᾧ�����롢����õ���Ʒ��
���������Ʒ���ȣ�
��1��������У���������Ҫ�ɷ���SiO2��
��2����MnO2����Fe2+�����ӷ���ʽ����������
1MnO2+2Fe2++4H+=1 Mn2++2Fe3++2H2O
��3����ѡ��Cl2��Ϊ��������ȣ�MnO2��������Ҫ���ڣ�ԭ����Դ�㡢�ɱ��͡��ɱ������Ⱦ����������Cl-��ʹ�Ƶõ�MnSO4?H2O��Ʒ��������
��4����֪��
�����������������pH
| Al��OH��3 | Fe��OH��2 | Fe��OH��3 | Mn��OH��2 | |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 | 7.6 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 | 10.2 |
�������a��ȡֵ��Χ��4.7��a��7.6��
��5���������ͨ���ⶨ��Ʒ����Ԫ�ص������������жϲ�Ʒ���ȣ�
��֪һ�������£�MnO4-��Mn2+��Ӧ����MnO2��ȡx g��Ʒ�����Һ����0.1mol/L KMnO4��Һ�ζ�������KMnO4��Һy mL����Ʒ����Ԫ�ص���������Ϊ$\frac{1.5y��1{0}^{-4}��55}{x}$��

��֪��
�ٽ�ȡʱ��������Ҫ��ӦΪ��MnO2+ZnS+2H2SO4=MnSO4+ZnSO4+S��+2H2O������FeS��CuS��CdSҲ�ᷢ�����Ʒ�Ӧ��
��ijЩ����������ȫ������pH���±���
| Zn2+ | Mn2+ | Fe2+ | Fe3+ | Al3+ | |
| pH | 8.0 | 10.1 | 9.0 | 3.2 | 4.7 |
��1��������н�ȡʱAl2O3��ϡ���ᷴӦ�Ļ�ѧ����ʽΪAl2O3+3H2SO4�TAl2��SO4��3+3H2O��
��2��������л�ԭ���յõ��Ľ���������Cu��Cd��
��3���������MnO2�������ǽ�Fe2+������Fe3+������ѡ������W���Լ�ΪBD��ѡ���ţ���
A��NaOH B��ZnO C��NH3•H2O D��MnCO3
��4������п�̸ɵ�طŵ�ʱ�������ĵ缫��ӦʽΪZn-2e-�TZn2+��
��5��MnSO4�������Ʊ�MnCO3��MnCO3�ڿ����м��ȷ�ӦҲ���Ƶ�MnO2��
��֪25�棬101kPaʱ��
Mn��s��+O2��g��=MnO2��s����H1=-520kJ•mol-1
C��s��+O2��g��=CO2��g����H2=-393.5kJ•mol-1
2Mn��s��+2C��s��+3O2��g��=2MnCO3��s����H3=-894kJ•mol-1
MnCO3�ڿ����м��ȷ�Ӧ����MnO2���Ȼ�ѧ����ʽΪ2MnCO3��s��+O2��g���T2MnO2��s��+2CO2��g����H=-933kJ•mol-1��
��6������ͼʾ���̣���ij������������ʹ����100t��п������ZnS����Ϊ80%����ȡʱZnS����ʧ��Ϊ3%�����յõ�87t MnO2������������ÿ�����趼��Ӧ��ȫ����⣨ʹ�ö��Ե缫��ʱ�������������ɣ��������г������������пԪ�ص�����Ϊ13t��
| ������ | K+��Na+��Fe2+��Ba2+��NH${\;}_{4}^{+}$��Ca2+ |
| ������ | OH-��NO${\;}_{3}^{-}$��I-��HCO${\;}_{3}^{-}$��AlO${\;}_{2}^{-}$��HSO${\;}_{4}^{-}$ |
��B�Ļ�ѧʽΪBa��OH��2��
��A��B��Һ��ϼ��ȷ�Ӧ�����ӷ���ʽH++SO42-+NH4++Ba2++2OH-$\frac{\underline{\;\;��\;\;}}{\;}$BaSO4��+NH3��+2H2O��
��2����A��ˮ��Һ��dz��ɫ��B��ˮ��Һ��ɫ������ɫ��ӦΪ��ɫ����A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ���A��B��ˮ��Һ����������Ա仯����
��A�Ļ�ѧʽΪFeI2��
�ھ�����������������Һ��Ƶ�ԭ����������֣������ӷ���ʽ��ʾ��
��8H++2NO3-+6I-=2NO��+3I2+4H2O����8H++2NO3-+6I-=2NO��+3I2+4H2O��4H++NO3-+3Fe2+=NO��+3Fe3++2H2O��
������һ������֤��������Һ��Ƶ�ԭ��ȡ���������Һ���Թ��У��μӼ���KSCN��Һ�������������������������ɣ���

| A�� | a�缫��ӦʽΪC6H5OH-28e-+11H2O�T6CO2��+28H+ | |
| B�� | ��������Ϊa�����ء�b�����ӽ���Ĥ��a | |
| C�� | H+������ͨ�����ӽ���Ĥ�������� | |
| D�� | ����1mol����ת��ʱ����������2.24L���� |



 ��
��