��Ŀ����
������Ӧ��������ѧ��ѧ�е���Ҫ��Ӧ���ڹ�ҵ������Ҳ����ҪӦ�ã���ʵ������������£�
����һ֧�Թ��м�������NaOH��Һ��������к���ȥ����������ˮϴ�����ã�
����ϴ�����Թ�������������Һ��
����������Һ�е���3��4����ȩϡ��Һ��
����ˮԡ�������Թ��г���������
��ش��������⣺
��1��������Ŀ���� ��
��2������������Һ�����ƹ��̣� ��
��3��д���������Ӧ�Ļ�ѧ����ʽ�� ��
��4��������Һ���ÿ��ܻᱬը��ֱ���ŷŻ���Ⱦ�������������Դ�˷ѣ�ʵ���Ҵӷ�Һ�л�������ʵ���������£���Һ
����
��֪��[Ag��NH3��2]+?Ag++2NH3
��д����Һ��ϡ���ᷴӦ�����ӷ���ʽ�� ��
�ڼ��������Ҫ������Ŀ���� ��
�۴ӻ����Ƕȿ��ǣ���ʵ���ȱ���� ��
����һ֧�Թ��м�������NaOH��Һ��������к���ȥ����������ˮϴ�����ã�
����ϴ�����Թ�������������Һ��
����������Һ�е���3��4����ȩϡ��Һ��
����ˮԡ�������Թ��г���������
��ش��������⣺
��1��������Ŀ����
��2������������Һ�����ƹ��̣�
��3��д���������Ӧ�Ļ�ѧ����ʽ��
��4��������Һ���ÿ��ܻᱬը��ֱ���ŷŻ���Ⱦ�������������Դ�˷ѣ�ʵ���Ҵӷ�Һ�л�������ʵ���������£���Һ
| ����ϡ���� |
| �������� |
| ����ϡ���� |
| ���ˡ�ϴ�� |
��֪��[Ag��NH3��2]+?Ag++2NH3
��д����Һ��ϡ���ᷴӦ�����ӷ���ʽ��
�ڼ��������Ҫ������Ŀ����
�۴ӻ����Ƕȿ��ǣ���ʵ���ȱ����
���㣺��ȩ��������Ӧ
ר�⣺
��������1����ʹ������Ӧʵ��ɹ�������NaOH��Һ����Թ��Գ�ȥ���ۣ����������������Ը������Թ��ϣ�
��2��������Һ���Ʊ�ע������Թ�Ҫ�ྻ����ϡ��ˮ�����������У�����������ij���ǡ���ܽ�Ϊֹ��
��3��������Ӧ�ķ���ʽ��д��ֻ��-CHO��2molAg��NH3��2OH��Ӧ����ƽ������1mol-CHO��2molAg��NH3��2OH��Ӧ��CH3CHO+2[Ag��NH3��2]OH
CH3COONH4+2Ag��+3NH3+H2O��
��4����������Һ�ijɷ�ΪAg��NH3��2OH����ˮ��ȫ�����룬�������[Ag��NH3��2]+��OH-��������ϡ����ʱ��H+���백�������������ӷ�Ӧ������ϡ���ᷴӦ�����ӷ���ʽΪ��[Ag��NH3��2]++OH-+3H+=Ag++2NH4++H2O��
�����������ã��ʼ�������ۿ���Ag+������Һ�л�ԭ���ӹ��������ۿ��Խ���Һ�е�Ag+ȫ����ԭ����������Ҫ������Ŀ����ʹ��Һ�е�Ag+ȫ����ԭ��
�۴���������Һ��һ���Ǽ���������ϡ���ᣬ�������������࣬������ɵ���������Ӧ��Ҳ������ڶ�����������۷�����Ӧ��ϡ����������ֽ���������Ӧ����NO�������ɣ�3Ag+4HNO3��ϡ��=3AgNO3+NO��+2H2O��Fe+4HNO3��ϡ��=3Fe��NO3��3+NO��+2H2O��
��2��������Һ���Ʊ�ע������Թ�Ҫ�ྻ����ϡ��ˮ�����������У�����������ij���ǡ���ܽ�Ϊֹ��
��3��������Ӧ�ķ���ʽ��д��ֻ��-CHO��2molAg��NH3��2OH��Ӧ����ƽ������1mol-CHO��2molAg��NH3��2OH��Ӧ��CH3CHO+2[Ag��NH3��2]OH
| ˮԡ |
��4����������Һ�ijɷ�ΪAg��NH3��2OH����ˮ��ȫ�����룬�������[Ag��NH3��2]+��OH-��������ϡ����ʱ��H+���백�������������ӷ�Ӧ������ϡ���ᷴӦ�����ӷ���ʽΪ��[Ag��NH3��2]++OH-+3H+=Ag++2NH4++H2O��
�����������ã��ʼ�������ۿ���Ag+������Һ�л�ԭ���ӹ��������ۿ��Խ���Һ�е�Ag+ȫ����ԭ����������Ҫ������Ŀ����ʹ��Һ�е�Ag+ȫ����ԭ��
�۴���������Һ��һ���Ǽ���������ϡ���ᣬ�������������࣬������ɵ���������Ӧ��Ҳ������ڶ�����������۷�����Ӧ��ϡ����������ֽ���������Ӧ����NO�������ɣ�3Ag+4HNO3��ϡ��=3AgNO3+NO��+2H2O��Fe+4HNO3��ϡ��=3Fe��NO3��3+NO��+2H2O��
���
�⣺��1����ʹ������Ӧʵ��ɹ�������NaOH��Һ����Թ��Գ�ȥ���ۣ����Թܲ��ྻ�����������Ը������Թ��ϣ��ʴ�Ϊ����ȥ�Թ��ڱڵ����ۣ���֤�Թܽྻ��
��2��������Һ���Ʊ�ע������Թ�Ҫ�ྻ����ϡ��ˮ�����������У�����������ij���ǡ���ܽ�Ϊֹ������ʱע����ྻ���Թ��м���1ml2%��AgNO3��Һ�У�Ȼ������Թܱ���ε���2%��ϡ��ˮ������������ij���ǡ����ʧΪֹ���ʴ�Ϊ����ྻ���Թ��м���1ml2%��AgNO3��Һ�У�Ȼ������Թܱ���ε���2%��ϡ��ˮ������������ij���ǡ����ʧΪֹ��
��3��������Ӧ�ķ���ʽ��д��ֻ��-CHO��2molAg��NH3��2OH��Ӧ�������ṹ���䣻��ƽ������1mol-CHO��2molAg��NH3��2OH��Ӧ����Ӧ�ķ���ʽΪCH3CHO+2[Ag��NH3��2]OH
CH3COONH4+2Ag��+3NH3+H2O���ʴ�Ϊ��CH3CHO+2[Ag��NH3��2]OH
CH3COONH4+2Ag��+3NH3+H2O��
��4����������Һ�ijɷ�ΪAg��NH3��2OH����ˮ��ȫ�����룬�������[Ag��NH3��2]+��OH-��������ϡ����ʱ��H+���백�������������ӷ�Ӧ������ϡ���ᷴӦ�����ӷ���ʽΪ��[Ag��NH3��2]++OH-+3H+=Ag++2NH4++H2O��
�ʴ�Ϊ��[Ag��NH3��2]++OH-+3H+=Ag++2NH4++H2O��
�����������ã��ʼ�������ۿ���Ag+������Һ�л�ԭ���ӹ��������ۿ��Խ���Һ�е�Ag+ȫ����ԭ����������Ҫ������Ŀ����ʹ��Һ�е�Ag+��Ӧ��ȫ���ʴ�Ϊ��ʹ��Һ�е�Ag+ȫ����ԭ��
�۴���������Һ��һ���Ǽ���������ϡ���ᣬ�������������࣬������ɵ���������Ӧ��Ҳ������ڶ�����������۷�����Ӧ��ϡ����������ֽ���������Ӧ����NO�������ɣ�3Ag+4HNO3��ϡ��=3AgNO3+NO��+2H2O��Fe+4HNO3��ϡ��=3Fe��NO3��3+NO��+2H2O���ʴ�Ϊ��������Ⱦ������NO��
��2��������Һ���Ʊ�ע������Թ�Ҫ�ྻ����ϡ��ˮ�����������У�����������ij���ǡ���ܽ�Ϊֹ������ʱע����ྻ���Թ��м���1ml2%��AgNO3��Һ�У�Ȼ������Թܱ���ε���2%��ϡ��ˮ������������ij���ǡ����ʧΪֹ���ʴ�Ϊ����ྻ���Թ��м���1ml2%��AgNO3��Һ�У�Ȼ������Թܱ���ε���2%��ϡ��ˮ������������ij���ǡ����ʧΪֹ��
��3��������Ӧ�ķ���ʽ��д��ֻ��-CHO��2molAg��NH3��2OH��Ӧ�������ṹ���䣻��ƽ������1mol-CHO��2molAg��NH3��2OH��Ӧ����Ӧ�ķ���ʽΪCH3CHO+2[Ag��NH3��2]OH
| ˮԡ |
| ˮԡ |
��4����������Һ�ijɷ�ΪAg��NH3��2OH����ˮ��ȫ�����룬�������[Ag��NH3��2]+��OH-��������ϡ����ʱ��H+���백�������������ӷ�Ӧ������ϡ���ᷴӦ�����ӷ���ʽΪ��[Ag��NH3��2]++OH-+3H+=Ag++2NH4++H2O��
�ʴ�Ϊ��[Ag��NH3��2]++OH-+3H+=Ag++2NH4++H2O��
�����������ã��ʼ�������ۿ���Ag+������Һ�л�ԭ���ӹ��������ۿ��Խ���Һ�е�Ag+ȫ����ԭ����������Ҫ������Ŀ����ʹ��Һ�е�Ag+��Ӧ��ȫ���ʴ�Ϊ��ʹ��Һ�е�Ag+ȫ����ԭ��
�۴���������Һ��һ���Ǽ���������ϡ���ᣬ�������������࣬������ɵ���������Ӧ��Ҳ������ڶ�����������۷�����Ӧ��ϡ����������ֽ���������Ӧ����NO�������ɣ�3Ag+4HNO3��ϡ��=3AgNO3+NO��+2H2O��Fe+4HNO3��ϡ��=3Fe��NO3��3+NO��+2H2O���ʴ�Ϊ��������Ⱦ������NO��
������������Ҫ�������������Һ�����Ƽ���ɷ֡�������Ӧʵ��ɹ��Ĺؼ����ء�������Һ��Һ�Ĵ����ȣ��ѶȲ���

��ϰ��ϵ�д�
 ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ
��0���1��105 Pa�£��ò��缫�������ͭ��Һ������������6.4gͭʱ�������ų���������
| A��1.12L���� |
| B��1.12L���� |
| C��2.24L���� |
| D��2.24L���� |
��ˮϡ��0.1Ħ/����ˮʱ����Һ������ˮ�������Ӷ���С���ǣ�������
A��
| ||
B��
| ||
| C��[H+]��[OH-]�ij˻� | ||
| D��OH-�����ʵ��� |
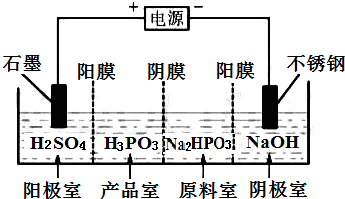 �����ᣨH3PO3����������NaOH��Һ��Ӧ����Na2HPO3��
�����ᣨH3PO3����������NaOH��Һ��Ӧ����Na2HPO3�� �״�������һ������Դ���ȼ�ϣ�������Ϊ���͵�������ҵ�Ͽ���CO��H2��ȡ�״�����ѧ����ʽΪ��
�״�������һ������Դ���ȼ�ϣ�������Ϊ���͵�������ҵ�Ͽ���CO��H2��ȡ�״�����ѧ����ʽΪ�� �ݱ�������һ�������£�N2�ڲ��������������Ķ������Ѵ�����������ˮ������Ӧ����Ҫ����ΪNH3����Ӧ�ķ�Ӧ����ʽΪ��2N2��g��+6H2O ��g��?4NH3��g��+3O2��g����H=Q kJ/mol
�ݱ�������һ�������£�N2�ڲ��������������Ķ������Ѵ�����������ˮ������Ӧ����Ҫ����ΪNH3����Ӧ�ķ�Ӧ����ʽΪ��2N2��g��+6H2O ��g��?4NH3��g��+3O2��g����H=Q kJ/mol