��Ŀ����
11�� ��֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ���ӣ�����֮�������ͼ��ʾ��ת����ϵ��
��֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ���ӣ�����֮�������ͼ��ʾ��ת����ϵ����1�����A��B��C��D����10���ӵ����ӣ���д����A�Ľṹʽ
 ��D�ĵ���ʽ
��D�ĵ���ʽ ��
����2�����A��C��18���ӵ����ӣ�B��D��10���ӵ����ӣ���д����
��A��B����Һ�з�Ӧ�����ӷ���ʽHS-+OH-�TS2-+H2O
�ڸ����������ӷ���ʽ�����ж�C��B������ӵ�������С�ǣ��û�ѧʽ�����ӷ��ű�ʾ��OH-��S2-��
��3����֪�£�H2N-NH2���ͼװ���CH3-NH2������18�����ӵķ��ӣ������ºͼװ��Ľṹ�ص㲢�����ܵ�������д�����������ͬ���������л�������Ľṹ��ʽ������д��������CH3CH3��CH3OH�ȣ�
���� ��1��10������A��B��Ӧ�õ�����10��������Ӧ��笠����������������ӷ�Ӧ�õ�������ˮ����C��B�����������ӷ�Ӧ������֪AΪNH4+��BΪOH-��CΪNH3��DΪH2O��
��2�����A��C����18���ӵ����ӣ�B��D��10�������ӣ����ת����ϵ�����ƶϣ�AΪH2S��BΪOH-��CΪHS-��S2-��DΪH2O��
��3���£�H2N-NH2���ͼװ���CH3-NH2������18�����ӵķ��ӣ���-NH2��-CH3��Ϊ9�����ӣ�-OHҲ��9�����ӣ���Ͽ��Եõ�������ͬ���������л������
��� �⣺��1��10������A��B��Ӧ�õ�����10��������Ӧ��笠����������������ӷ�Ӧ�õ�������ˮ����C��B�����������ӷ�Ӧ������֪AΪNH4+��BΪOH-��CΪNH3��DΪH2O��NH4+�ĽṹʽΪ
 ��H2O�ĵ���ʽΪ
��H2O�ĵ���ʽΪ ��
��
�ʴ�Ϊ�� ��
�� ��
��
��2�����A��C����18���ӵ����ӣ�B��D��10�������ӣ����ת����ϵ�����ƶϣ�AΪH2S��BΪOH-��CΪHS-��S2-��DΪH2O��A��B����Һ�з�Ӧ�����ӷ���ʽΪ��HS-+OH-�TS2-+H2O���������ӷ���ʽ�������жϽ�����ӵ�������С��OH-��S2-��
�ʴ�Ϊ��HS-+OH-�TS2-+H2O��OH-��S2-��
��3���£�H2N-NH2���ͼװ���CH3-NH2������18�����ӵķ��ӣ���-NH2��-CH3��Ϊ9�����ӣ�-OHҲ��9�����ӣ��Եõ�������ͬ���������л�������ΪCH3CH3��CH3OH�ȣ�
�ʴ�Ϊ��CH3CH3��CH3OH�ȣ�
���� ���⿼�������ƶϣ��������ճ���10���ӡ�18�������Ľṹ������Ӧ�ã��Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 17g�ǻ���17g NH3������������Ϊ10NA | |
| B�� | lmolCl2����ˮת�Ƶ�����ΪNA | |
| C�� | 1L0.1mol•L-1 NH4 HC03��Һ������NH4+��Ϊ0.1NA | |
| D�� | ��״���£�11.2LN2��02�������������������Ϊ0.5NA |
| A�� | ԭ��������a��b��c��d | |
| B�� | ���Ӱ뾶��A��n+1��+��Bn+��C��n+1��-��Dn- | |
| C�� | ���������ԣ�A��n+1��+��Bn+���ӻ�ԭ�ԣ�C��n+1��-��Dn- | |
| D�� | ���ʻ�ԭ�ԣ�A��B�����������ԣ�D��C |
��֪��
��
 | 10 | 20 | 30 | 40 | 50 | 70 |
| ��NH4��2SO4 | 73.3 | 75.4 | 78.0 | 81.0 | 84.5 | 91.9 |
| FeSO4•7H2O | 40.0 | 48.0 | 60.0 | 73.3 | - | - |
| ��NH4��2SO4•FeSO4•6H2O | 18.1 | 21.2 | 24.5 | 27.9 | 31.3 | 38.5 |
��Ī���ε���ȡ

�Է�����
��1������2�м��ȷ�ʽ ���ֱ�Ӽ��ȡ���ˮԡ���ȡ���ɳԡ��������������м����ʣ��ʱ�������ȹ��ˣ���ԭ���Ƿ�ֹFe2+��������ͬʱ�ȹ��˿ɷ�ֹ���������Ծ�����ʽ������
��2������3�а�����ʵ��������Ƽ���Ũ������ȴ�ᾧ��
��3����ƷĪ���������bϴ�ӣ�����ĸ��ţ���
a������ˮ b���Ҵ� c����Һ
��Ϊ�ⶨ��������泥�NH4��2SO4FeSO46H2O���崿�ȣ�ijѧ��ȡm g�����������Ʒ���Ƴ�500mL��Һ������������ɣ��ס��ҡ�����λͬѧ�������������ʵ�鷽������ش�
���ף�����һ��ȡ20.00mL�����������Һ��0.1000molL -1������KMnO4��Һ�����ν��еζ���
���ң���������ȡ20.00mL�����������Һ��������ʵ�飮

��1����ʵ���������ȷ��������һ�IJⶨ�������С�ڷ������������ԭ��ΪFe2+�ѱ�����������������֤�Ʋ�ķ���Ϊ��ȡ���������������Һ����������KSCN��Һ������Һ��ΪѪ��ɫ��˵��Fe2+�ѱ���������������
����������������ͨ��NH 4+�ⶨ��ʵ�����ͼ������ʾ��ȡ20.00mL�����������Һ���и�ʵ�飮

��2����װ���ң���ס����ҡ�����Ϊ�������ж������Ǽ�װ�û���ֵ�����������������Լ��� ������ĸ��ţ���ѡ���ҡ�����˿գ���ѡ���ס��˿տɲ����
a��ˮ b������NaHCO3��Һ c��CCl4
�������NH3�����ΪV L��������Ϊ��״���£��������������茶���Ĵ���Ϊ$\frac{392V��25}{44.8m}$��100%��
 þȼ�ϵ�ؾ��б������ߡ�ʹ�ð�ȫ���㡢ԭ������Դ�ḻ���ɱ��͵��ص㣮һ���о���þȼ�ϵ�ؿɷ�Ϊþ����ȼ�ϵ�ء�þ��ˮȼ�ϵ�ء�þ��������ȼ�ϵ�غ�þ��������ȼ�ϵ�أ����У�þ��������ȼ�ϵ�صĹ���ԭ����ͼ��ʾ�������й�˵������ȷ���ǣ�������
þȼ�ϵ�ؾ��б������ߡ�ʹ�ð�ȫ���㡢ԭ������Դ�ḻ���ɱ��͵��ص㣮һ���о���þȼ�ϵ�ؿɷ�Ϊþ����ȼ�ϵ�ء�þ��ˮȼ�ϵ�ء�þ��������ȼ�ϵ�غ�þ��������ȼ�ϵ�أ����У�þ��������ȼ�ϵ�صĹ���ԭ����ͼ��ʾ�������й�˵������ȷ���ǣ�������| A�� | �ŵ������OH-�������� | |
| B�� | ��ص��ܷ�ӦʽΪMg+ClO-+H2O�TMg��OH��2+Cl- | |
| C�� | þȼ�ϵ����þ��Ϊ����������������Ӧ | |
| D�� | þ��������ȼ�ϵ�أ����Ե������������ӦʽΪH2O2+2H++2e-�T2H2O |
 ���ش��������⣺
���ش��������⣺

 ��
�� ��
�� ��
��
 ���ڴ������������ɾ۱���ϩ�ķ�Ӧ����ʽ��
���ڴ������������ɾ۱���ϩ�ķ�Ӧ����ʽ��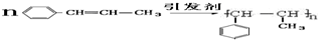
 +3HNO3$��_{��}^{Ũ����}$
+3HNO3$��_{��}^{Ũ����}$ +3H2O��
+3H2O��