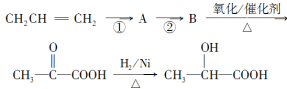��Ŀ����
����Ŀ���л��߷��ӻ��������һ�ֳ��õĹ����߷��Ӳ��ϣ���ṹ��ʽΪ
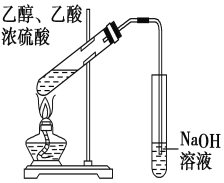
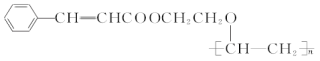 ������ͼ��ʾ��ϵ���Ժϳɼף������Լ�I�����Ҵ��������ڴ������������·�Ӧ�õ���
������ͼ��ʾ��ϵ���Ժϳɼף������Լ�I�����Ҵ��������ڴ������������·�Ӧ�õ���
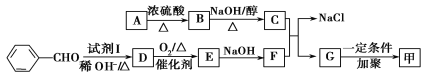
��֪��
a����CH2OH �� ��CH2OH![]() ��CH2OCH2�� �� H2O
��CH2OCH2�� �� H2O
��ش��������⣺
��1������ͼ��ʾA����Է���������80.5��A��������Ԫ�ص���������Ϊ19.88%��̼Ԫ�ص���������Ϊ29.81%������Ϊ��Ԫ�غ���Ԫ�أ���A�ĺ˴Ź������������������շ壬�����֮��Ϊ2��2��1����A�Ľṹ��ʽΪ_______________________________________��
��2���Լ�I��������__________________��B��C�ķ�Ӧ������__________________��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
��D��E___________________________________________________________________��
��C��F��G��NaCl_________________________________________________________��
��4��E��һ��ͬ���칹���ˮ����������֣�һ����ʹ��ˮ��ɫ����һ���ڵμӱ�����ˮ���а�ɫ�������ɣ������ʵĽṹ��ʽΪ______________________________________��
���𰸡�
��1��ClCH2CH2OH��
��2����ȩ����ȥ��Ӧ��
��3����2 ![]()
![]() ����
����![]()
![]()
��4�� ![]() ��
��
��������
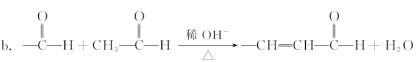
�����������1������������Ϣa���Լ���Ӧ�������Ƴ�A�к����ǻ��������л���ķ��������Ҵ��ķ����������Աȣ������ʽΪC2H5OCl���˴Ź��������������ַ壬�ҷ����֮��Ϊ2��2��1����ṹ��ʽΪClCH2CH2OH����2���Լ�I���Ҵ��������ڴ������µõ��ģ������Լ�IΪ��ȩ��B��C��Ӧ��������NaOH�Ĵ���Һ����Ҫ���ȣ�����±��ԭ�ӷ�����ȥ��Ӧ������������Ӧ����Ϊ��ȥ��Ӧ����3����������Ϣb���Ƴ�D�Ľṹ��ʽ��![]() ��ȩ���ڴ����������£����������Ȼ����䷴Ӧ����ʽΪ��2
��ȩ���ڴ����������£����������Ȼ����䷴Ӧ����ʽΪ��2 ![]()
![]() ����E��NaOH�����кͷ�Ӧ����F�Ľṹ��ʽΪ��
����E��NaOH�����кͷ�Ӧ����F�Ľṹ��ʽΪ��![]() ����˷�Ӧ����ʽΪ��
����˷�Ӧ����ʽΪ��![]()
![]() ��4����ˮ��˵������������һ����ʹ��ˮ��ɫ��˵������̼̼�����ͼ����μӱ�����ˮ�а�ɫ�������ɣ�˵�����з��ǻ�����˸����ʵĽṹ��ʽΪ��
��4����ˮ��˵������������һ����ʹ��ˮ��ɫ��˵������̼̼�����ͼ����μӱ�����ˮ�а�ɫ�������ɣ�˵�����з��ǻ�����˸����ʵĽṹ��ʽΪ��![]() ��
��