��Ŀ����
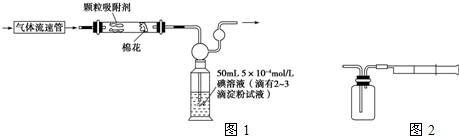
��ҵ�ϲ���������������������������ж�����������װ������ͼ��ʾ����Ӧ����װ�е�ĵ�����Һ����������͵ⷢ���ķ�ӦΪ����������������ⷴӦ����SO2+I2+2H2O=H2SO4+2HI

(1)���������뷴Ӧ�ܺ������������ӵ�ˮ��������� ______________����д��ѧʽ���������
(2)��Ӧ������Һ��ɫ��ʧ��û�м�ʱֹͣͨ�������õĶ���������___________���ƫ�ߡ���ƫ�͡�����Ӱ�족����
(3)��Ӧ���ڵĵ�ĵ�����ҺҲ������__________ ����д�������ƣ����档
(4)������Һ���ΪVamL��Ũ��Ϊc mol��L-1��N2��O2�����ΪVbmL(������Ϊ��״���µ����)����c��Va��Vb�� ʾ����������������Ϊ___________��
(5)������װ�ø�Ϊ����ʵ��װ�ã��������⣬����ѡ�õ�����Ϊ__________�������������ı�ţ���
a.�ձ� b���Թ� c�����ƿ d������ƿ e����Ͳ f.������ g��˫����
(2)��Ӧ������Һ��ɫ��ʧ��û�м�ʱֹͣͨ�������õĶ���������___________���ƫ�ߡ���ƫ�͡�����Ӱ�족����
(3)��Ӧ���ڵĵ�ĵ�����ҺҲ������__________ ����д�������ƣ����档
(4)������Һ���ΪVamL��Ũ��Ϊc mol��L-1��N2��O2�����ΪVbmL(������Ϊ��״���µ����)����c��Va��Vb�� ʾ����������������Ϊ___________��
(5)������װ�ø�Ϊ����ʵ��װ�ã��������⣬����ѡ�õ�����Ϊ__________�������������ı�ţ���
a.�ձ� b���Թ� c�����ƿ d������ƿ e����Ͳ f.������ g��˫����
(1) N2 ��O2
(2)ƫ��
(3)���Ը��������Һ
(4)
(5)b��c��e��g��b.e.g��c��e��g���𰸲�Ψһ��
(2)ƫ��
(3)���Ը��������Һ
(4)

(5)b��c��e��g��b.e.g��c��e��g���𰸲�Ψһ��

��ϰ��ϵ�д�
 �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ