��Ŀ����
�����������ʣ����ö�Ӧ�Ļ�ѧ����ͽ�����գ�
���ʯ �������� �������� Al �ɱ� �� ��� N2 CH4
��1��ͨ���Ǽ��Լ��γɵ�ԭ�Ӿ�����ӻ��������Ϊ ��
��2����N2��Ϊ�ȵ�����ij���������ˮ�е��ܽ��Ա�N2 ������С������
��3��������ѧ���ķ��Ӿ��壬�����Ԫ�������ڱ��е�λ��Ϊ�� ���� �壮
��4��Al�ܵ����ԭ����
��5��д����ҵ�����ö���������ȡ�ֹ�ķ�Ӧ����ʽ�������˫���ű�ʾe-ת�Ƶķ������Ŀ��
��6��ˮ����������ԭ�� ��
���ʯ �������� �������� Al �ɱ� �� ��� N2 CH4
��1��ͨ���Ǽ��Լ��γɵ�ԭ�Ӿ�����ӻ��������Ϊ
��2����N2��Ϊ�ȵ�����ij���������ˮ�е��ܽ��Ա�N2
��3��������ѧ���ķ��Ӿ��壬�����Ԫ�������ڱ��е�λ��Ϊ��
��4��Al�ܵ����ԭ����
��5��д����ҵ�����ö���������ȡ�ֹ�ķ�Ӧ����ʽ�������˫���ű�ʾe-ת�Ƶķ������Ŀ��
��6��ˮ����������ԭ��
���㣺ԭ�ӹ���ӻ���ʽ���ӻ������ж�,Ԫ�����ڱ��Ľṹ����Ӧ��,���ȵ���ԭ������Ӧ��,�������������,��Ͷ�������,������ͨ��
ר�⣺��ѧ���뾧��ṹ
��������1�����ʯ����ԭ�Ӿ��壬̼ԭ�Ӽ�ͨ���Ǽ��Լ���ϣ�����̼�γɵĹ��ۼ��ж��ӻ����ͣ�
��2��CO��N2��Ϊ�ȵ����壬������������ԭ���ж�������ܽ��ԣ�
��3��ϡ������Ϊ��ԭ�ӷ��ӣ�
��4�������д������ɵ��ӣ�
��5��д����ѧ����ʽ�����û��ϼ۵ı仯����������ת�Ƶ���Ŀ��
��6������ˮ����֮����������
��2��CO��N2��Ϊ�ȵ����壬������������ԭ���ж�������ܽ��ԣ�
��3��ϡ������Ϊ��ԭ�ӷ��ӣ�
��4�������д������ɵ��ӣ�
��5��д����ѧ����ʽ�����û��ϼ۵ı仯����������ת�Ƶ���Ŀ��
��6������ˮ����֮����������
���
�⣺��1�����ʯ����ԭ�Ӿ��壬̼ԭ�Ӽ�ͨ���Ǽ��Լ���ϣ�ÿ��̼ԭ���γɵ�4�����ۼ���������4���ɼ����Ӷԣ�����sp3�ӻ����ʴ�Ϊ��sp3��
��2��CO��N2��Ϊ�ȵ����壬COΪ���Է��ӣ������ڼ����ܼ�ˮ��N2Ϊ�Ǽ��Է��ӣ���ˮ�е��ܽ��С���ʴ�Ϊ����
��3��ϡ������Ϊ��ԭ�ӷ��ӣ�������û�й��ۼ����������û�л�ѧ�����������ڱ���λ��Ϊ��������0�壬�ʴ�Ϊ������0��
��4�������д������ɵ��ӣ�����ӵ糡�£����ɵ��Ӷ����ƶ����γɵ������ܵ��磬�ʴ�Ϊ������ӵ糡�£����ɵ��Ӷ����ƶ����γɵ������ܵ��磻
��5����ҵ�϶���������ȡ�ֹ�ķ�Ӧ����ʽΪSiO2+2C
Si+CO����1molSiO2ת��4mol���ӣ���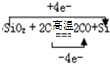 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��6������ˮ����֮����������ˮ���̺��������������ʹ�����������ĵ�ÿһ��ˮ�����������嶥�Ƿ����4�����ڵ�ˮ������������γ�������ṹ���ռ������ʵͣ����нϴ�ռ䣬
�ʴ�Ϊ�����̺��������������ʹ�����������ĵ�ÿһ��ˮ�����������嶥�Ƿ����4�����ڵ�ˮ������������γ�������ṹ���ռ������ʵͣ����нϴ�ռ䣮
��2��CO��N2��Ϊ�ȵ����壬COΪ���Է��ӣ������ڼ����ܼ�ˮ��N2Ϊ�Ǽ��Է��ӣ���ˮ�е��ܽ��С���ʴ�Ϊ����
��3��ϡ������Ϊ��ԭ�ӷ��ӣ�������û�й��ۼ����������û�л�ѧ�����������ڱ���λ��Ϊ��������0�壬�ʴ�Ϊ������0��
��4�������д������ɵ��ӣ�����ӵ糡�£����ɵ��Ӷ����ƶ����γɵ������ܵ��磬�ʴ�Ϊ������ӵ糡�£����ɵ��Ӷ����ƶ����γɵ������ܵ��磻
��5����ҵ�϶���������ȡ�ֹ�ķ�Ӧ����ʽΪSiO2+2C
| ||
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����6������ˮ����֮����������ˮ���̺��������������ʹ�����������ĵ�ÿһ��ˮ�����������嶥�Ƿ����4�����ڵ�ˮ������������γ�������ṹ���ռ������ʵͣ����нϴ�ռ䣬
�ʴ�Ϊ�����̺��������������ʹ�����������ĵ�ÿһ��ˮ�����������嶥�Ƿ����4�����ڵ�ˮ������������γ�������ṹ���ռ������ʵͣ����нϴ�ռ䣮
���������⿼�龧������ͼ������Ļ�ѧ�����ࡢ����ת�ơ���������ԭ��������ȣ���Ŀ�Ѷ��еȣ�Ҫע��ϡ������Ϊ��ԭ�ӷ��ӣ������л�ѧ����

��ϰ��ϵ�д�
�����Ŀ
���и���Һ�е�����������Ũ����ͬʱ�����ʵ���Ũ�������ǣ�������
| A��NH3?H2O |
| B��NaOH |
| C��KOH |
| D��Ba��OH��2 |
 ��Ҫ��ش���������
��Ҫ��ش���������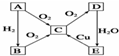 ��֪��AΪ�����к������ĵ��ʣ�������ͼת����ϵ���ش��������⣺
��֪��AΪ�����к������ĵ��ʣ�������ͼת����ϵ���ش��������⣺