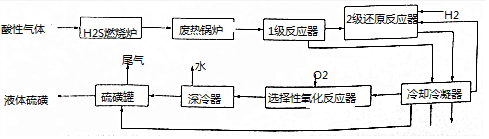
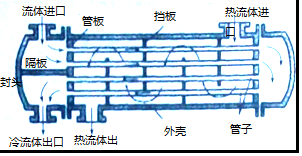
��Ŀ����
7����֪һ��̼ԭ�������������ǻ�ʱ����������ת����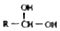 $\stackrel{-H_{2}O}{��}$
$\stackrel{-H_{2}O}{��}$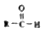 �������ͼ�ش�
�������ͼ�ش�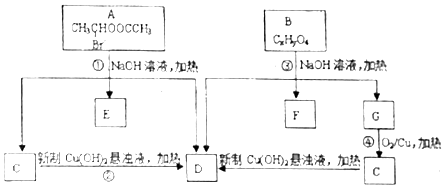
��1��A�����������ŵ�����Ϊ��������ԭ�ӣ�
��2�������Ƿ�������B������ʺɱ�Ϊ208�����������ʾB�����к��б����ṹ�������������˴Ź�����������������շ壬���ֵ��Ϊ2��2��2��3��3�����б����ϵ�һ�ȴ���ֻ�����֣���B�Ľṹ��ʽΪ
 ��
����3��д�����з�Ӧ����ʽ��
��
 ����CH3CHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$CH3COONa+Cu2O+3H2O��
����CH3CHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$CH3COONa+Cu2O+3H2O����4����������������B��ͬ���칹�干��9�֣�
�����ڷ����廯����
�ں�������ȡ����������ֻ��һ���ǻ���������ȡ������ͬ�Ҵ�����ͬ��λ��
���ܷ���ˮ�ⷴӦ��������Ӧ
��5����֪��2RCH2COOC2H3$\stackrel{Na}{��}$
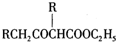 +C2H3OH������GΪΨһ�л��Լ��ϳ���������������CH3COCH2COOC2H5������ƺϳ�·�ߣ������Լ���ѡ����
+C2H3OH������GΪΨһ�л��Լ��ϳ���������������CH3COCH2COOC2H5������ƺϳ�·�ߣ������Լ���ѡ�����ϳ�·������ͼʾ����CH3CH2Cl$��_{��}^{NaOH��Һ}$CH3CHOH$��_{Ũ����/��}^{CH_{3}COOH}$CH3COOC2H��
���� A����ˮ�ⷴӦC��D��E��C������D������������Ϣ��֪��C�Ľṹ��ʽΪCH3CHO��DΪCH3COONa��EΪNaBr��B������ʺɱ�Ϊ208������B����Է�������Ϊ208�����������ʾB�����к��б����ṹ���������������б����ϵ�һ�ȴ���ֻ�����֣��˴Ź�����������������շ壬���ֵ��Ϊ2��2��2��3��3��B����ˮ���D��G��G������C��C������D����BΪ ��FΪ
��FΪ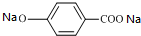 ��GΪCH3CH2OH���Դ˽����⣮
��GΪCH3CH2OH���Դ˽����⣮
��� �⣺A����ˮ�ⷴӦC��D��E��C������D������������Ϣ��֪��C�Ľṹ��ʽΪCH3CHO��DΪCH3COONa��EΪNaBr��B������ʺɱ�Ϊ208������B����Է�������Ϊ208�����������ʾB�����к��б����ṹ���������������б����ϵ�һ�ȴ���ֻ�����֣��˴Ź�����������������շ壬���ֵ��Ϊ2��2��2��3��3��B����ˮ���D��G��G������C��C������D����BΪ ��FΪ
��FΪ ��GΪCH3CH2OH��
��GΪCH3CH2OH��
��1��A�����������ŵ�����Ϊ��������ԭ�ӣ��ʴ�Ϊ����������ԭ�ӣ�
��2��ͨ�����Ϸ���֪��B�Ľṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��Ӧ�ٵķ���ʽΪ ����Ӧ�ڵķ���ʽΪCH3CHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$CH3COONa+Cu2O+3H2O��
����Ӧ�ڵķ���ʽΪCH3CHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$CH3COONa+Cu2O+3H2O��
�ʴ�Ϊ�� ��CH3CHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$CH3COONa+Cu2O+3H2O��
��CH3CHO+2Cu��OH��2+NaOH$\stackrel{��}{��}$CH3COONa+Cu2O+3H2O��
��4��BΪ ���������������ڷ����廯���˵���б������ں�������ȡ����������ֻ��һ��������������ȡ������ͬ�Ҵ�������λ�ã����ܷ���ˮ�ⷴӦ��������Ӧ��˵����������ȩ���������������B��ͬ���칹��Ϊ�ڱ����ļ�λ��������HCOO-��һ��-C3H7�����������з�������-C3H7��2�ֽṹ�����Թ���6�ֽṹ��Ҳ�������ڱ����ļ�λ��������HCOOCH2-��һ��-CH3�����������з��������Թ���9�֣�
���������������ڷ����廯���˵���б������ں�������ȡ����������ֻ��һ��������������ȡ������ͬ�Ҵ�������λ�ã����ܷ���ˮ�ⷴӦ��������Ӧ��˵����������ȩ���������������B��ͬ���칹��Ϊ�ڱ����ļ�λ��������HCOO-��һ��-C3H7�����������з�������-C3H7��2�ֽṹ�����Թ���6�ֽṹ��Ҳ�������ڱ����ļ�λ��������HCOOCH2-��һ��-CH3�����������з��������Թ���9�֣�
�ʴ�Ϊ��9��
��5����CH3CH2OHΪԭ�Ϻϳ���������������CH3COCH2COOC2H5�������������Ҵ����������ᣬ�Ҵ����Ҵ����������������������������Ƶ������·���������Ϣ�еķ�Ӧ�����������������ϳ�·��Ϊ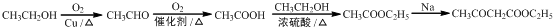 ��
��
�ʴ�Ϊ��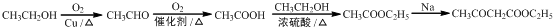 ��
��
���� ���⿼���л�����ƶϣ�ע�����ȷ��B�ķ���ʽ���ٸ����л���Ĺ����ŵı仯Ϊͻ�ƿڽ����ƶϣ���Ҫѧ���Ը������Ϣ�������ã���Ҫѧ���������չ����ŵ�������ת�����Ѷ��еȣ�

 ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�| A�� | ��������ˮ��Һ����ǿ�����ԣ��ʿ����������� | |
| B�� | �������ʴ�̲����������������������� | |
| C�� | �����£����ܱ�Ũ����ۻ���������������Ũ���� | |
| D�� | ��¯ˮ���к��е�CaSO4��������Na2CO3��Һ�������������ȥ |
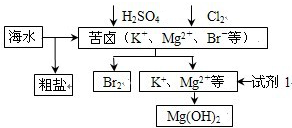
| A�� | �Լ�1����ѡ��ʯ���� | |
| B�� | �ӿ�±����ȡBr2�ķ�Ӧ�����ӷ���ʽΪ��2Br-+Cl2=2 Cl-+Br2 | |
| C�� | ��ҵ�ϣ��������MgOұ������þ�ɼ�С�ܺ� | |
| D�� | ���ξ��ᴿ��������Ʊ��������ƵȲ�Ʒ |
| A�� | ��������������ˮ������ | B�� | ���������ǰ뵼����� | ||
| C�� | �����������������ھ�ˮ | D�� | �������������Ư��ֽ�� |
| A�� | pH=1��H2SO4��Һ����H+����ĿΪ2NA | |
| B�� | 1mol Na������O2��Ӧ������Na2O��Na2O2�Ļ�����ʧȥNA������ | |
| C�� | 273K��101kPa�£�14g��ϩ���ϩ������к���̼ԭ����ĿΪ3NA | |
| D�� | 0.2mol C2H6O������һ������0.2NA��̼̼���� |
| A�� | CaO ��CO2 | B�� | NaCl��HCI | C�� | SiC ��SiO2 | D�� | CCl4��I2 |
 ����ͼ��ת����ϵ�У���֪A���ɶ�����Ԫ����ɵ���ʽ�Σ�D��Y��HΪ���壬XΪ��ɫҺ�壬G��K���dz�����ǿ�ᣮH��Na2O2�ɷ������Ϸ�Ӧ�����ɵ�����Ba2+��Ӧ�����ɲ�����ϡG�İ�ɫ������һ��D�����к���10�����ӣ�
����ͼ��ת����ϵ�У���֪A���ɶ�����Ԫ����ɵ���ʽ�Σ�D��Y��HΪ���壬XΪ��ɫҺ�壬G��K���dz�����ǿ�ᣮH��Na2O2�ɷ������Ϸ�Ӧ�����ɵ�����Ba2+��Ӧ�����ɲ�����ϡG�İ�ɫ������һ��D�����к���10�����ӣ� ��
��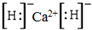 ���������ӻ��������ˮ��Ӧ����ѧ����ʽΪCaH2+2H2O=Ca��OH��2+2H2����
���������ӻ��������ˮ��Ӧ����ѧ����ʽΪCaH2+2H2O=Ca��OH��2+2H2����