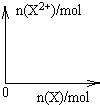��Ŀ����
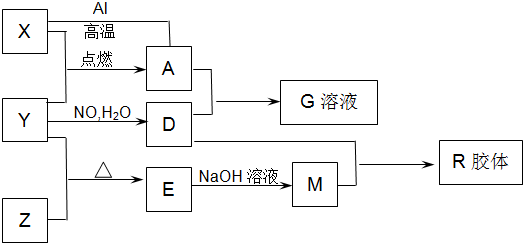
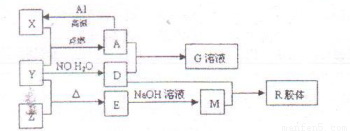
ͼ��X��Y��ZΪ���ʣ�����Ϊ��ѧ�����֮���������ת����ϵ�����ֲ�������ȥ�������У�A�׳ƴ�����������E�Dz�����ˮ�������������������ᷴӦ��

�ش��������⣺
��1����ɵ���Y��Ԫ�������ڱ��е�λ����__________��M�д��ڵĻ�ѧ������Ϊ__________��R�Ļ�ѧʽ��__________��
��2��һ�������£�Z��H2��Ӧ����ZH4��ZH4�ĵ���ʽΪ__________��
��3����֪A��1molAl��Ӧת��ΪXʱ���������ʾ�Ϊ���壩���ų�aKJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��__________________________��
��4��д��A��D��ϡ��Һ��Ӧ����G�����ӷ���ʽ��____________________��
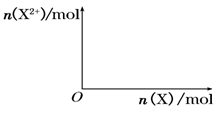
��5����4mol D��ϡ��Һ�У�����X��ĩ���������������ɵ�����ֻ��һ�֣���������ϵ�л���
n(X2+)��n��X���仯��ʾ��ͼ�������n(X2+)�����ֵ��
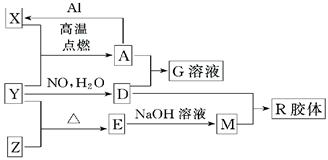
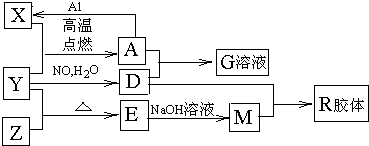
��1����ɵ���Y��Ԫ�������ڱ��е�λ����__________��M�д��ڵĻ�ѧ������Ϊ__________��R�Ļ�ѧʽ��__________��
��2��һ�������£�Z��H2��Ӧ����ZH4��ZH4�ĵ���ʽΪ__________��
��3����֪A��1molAl��Ӧת��ΪXʱ���������ʾ�Ϊ���壩���ų�aKJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��__________________________��
��4��д��A��D��ϡ��Һ��Ӧ����G�����ӷ���ʽ��____________________��
��5����4mol D��ϡ��Һ�У�����X��ĩ���������������ɵ�����ֻ��һ�֣���������ϵ�л���
n(X2+)��n��X���仯��ʾ��ͼ�������n(X2+)�����ֵ��

��1���ڶ����ڵڢ�A�壻���Ӽ������ۼ���H2SiO3��H4SiO4��
��2��

��3��8Al(s)��3Fe3O4(s)��9Fe(s)��4Al2O3(s) ��H��-8aKJ/mol
��4��3Fe3O4��28H+��NO3-=9Fe3+��14H2O��NO��
��4��3Fe3O4��28H+��NO3-=9Fe3+��14H2O��NO��
��5��


��ϰ��ϵ�д�
�����Ŀ


 9Fe3++NO��+14H2O
9Fe3++NO��+14H2O