��Ŀ����
13����嫵Ĵ��̲��ŷḻ�Ļ�ѧ��Դ�����������ú�ˮ��Դ�ǵ�ǰ��ѧ�о���һ����Ҫ������ͼ��ij�������Ժ�ˮ��Դ�ۺ����õ�ʾ��ͼ��
�����������Ϣ�ش��������⣺
��1��д������ڷ����ᴿ�ķ��������ˣ�
��2������ݷ����ᴿ��������Ҫѡ�õIJ����������ձ��⣬����Ҫ�������Ƿ�Һ©��
��3�������ᴿ��Ĵ����к���Ca2+��Mg2+��SO42-�����ʣ�����ʱ���õ��Լ�Ϊ���˿�ɾȥ
��������Ȼ�����Һ������������Һ��̼������Һ��
�����Լ�����˳���������AD��
A���ڢۢܢ�B���ۢܢڢ�
C���ܢۢڢ�D���ۢڢܢ�
��4����Ӧ���е�������Cl2��;�㷺����д��Cl2��NaOH ��Һ����Ư��Һ�ķ���ʽCl2+2NaOH=NaCl+NaClO+H2O��
��5�����̢ܽ��嵥�ʴ�ˮ��Һ�з�������ǻ����嵥�ʾ��лӷ��ԣ��÷����Ա��л��ܼ���ȡ�����ŵ��Ǿ��ã����������㣮
���� ��ˮͨ������Ũ���õ����κ�±ˮΪĸҺ������ͨ�����˳�ȥ��ɳ�õ������ᴿ�Ĵ��Σ������Լ���ȥ�������Ӿ��Ƶõ����Σ�ĸҺ�к��������ӡ�þ���ӵȣ�������������ˮ����������Ϊ�嵥�ʣ������嵥�ʾ����ӷ������ȿ��������嵥�ʣ��õ����壻���������ܽ�õ��������ӵ�ˮ��Һ����������������������Ϊ�ⵥ�ʣ������л���ȡ����ȡ�ⵥ�ʣ���Һ����л�������õ��ⵥ�ʣ�
��1���������õ��������ж�ʹ�ò�����������������������֪���������Ƿ�����Һ���壬�ù��˲�����
��2������ݷ����ᴿ��������ȡ��Һ����Ҫѡ����Ҫ��������Ϊ��Һ©�����ձ���
��3��þ���������������ӳ���������������ñ����ӳ�������������̼������ӳ���������Ҫ�������еij�������֮��̼����Ҫ���ڼ��Ȼ���֮���Խ������ı����ӳ�������������ᴦ����Һ�е�̼������Ӻ����������ӽ��з�����
��4������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ��
��5�������ӷ���Һ�壬���̢��嵥�ʴ�ˮ��Һ�з�����������������ȿ�����ʹ�嵥�ʻӳ����Ա��л��ܼ���ȡ��������Ҫ�Լ�������Ⱦ���������豸���㣮
��� �⣺��1���������Ƿ�����Һ���壬�ù��˲��������Բ����Ϊ���ˣ�
�ʴ�Ϊ�����ˣ�
��2������ݷ����ᴿ����Ϊ��ȡ����Ҫѡ����Ҫ��������Ϊ��Һ©�����ձ���
�ʴ�Ϊ����Һ©����
��3�����������ᾧ�ķ������ԴӺ�ˮ�л��ʳ�Σ�þ���������������ӳ��������������ʯ������Խ�þ���ӳ���������������ñ����ӳ���������������Ȼ������Խ���������ӳ����������ȳ�þ���ӣ������ȳ���������Ӷ��У���������̼������ӳ������������Ӽ���̼����ת��Ϊ���������Ǽ����̼����Ҫ���ڼ����Ȼ���֮������̼���ƻ��ȥ��Ӧʣ����Ȼ��������Ӷ������ˣ��ڽ��й��ˣ�����ټ��������ȥ��Ӧʣ������������Ӻ�̼������ӣ����������Լ���˳��Ϊ�ڢۢܢٻ�ۢڢܢ٣�
�ʴ�Ϊ��AD��
��4���������������Ʒ�Ӧ���ɴ����ᡢ�Ȼ��ƺ�ˮ����Һ�Ļ�ѧ����ʽΪ��Cl2+2NaOH=NaCl+NaClO+H2O��
�ʴ�Ϊ��Cl2+2NaOH=NaCl+NaClO+H2O��
��5���嵥�ʾ��лӷ��ԣ����嵥�ʴӻ�����з�������ǻ����嵥�ʵĻӷ��ԣ��÷����Ա��л��ܼ���ȡ�����ŵ��Dz���Ҫ�Լ�������Ⱦ���������豸���㣬
�ʴ�Ϊ���ӷ������ã����������㣮
���� ���⿼���˺�ˮ��Դ���ۺ����ã�������ѧ���ķ���������ʵ�������Ŀ��飬���ֻ�ѧ��Դ������������ַ���������������������Ŀ�Ѷ��еȣ�

| A�� | I-131��${\;}_{53}^{77}$I | B�� | Cl-�Ľṹʾ��ͼ��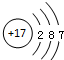 | ||
| C�� | ��Ȳ�Ľṹ��ʽ��CHCH | D�� | Na2S�ĵ���ʽ��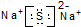 |
| A�� | �����ԣ�F2��Cl2��Br2��I2 | |
| B�� | ��������ɱ��������������������������ˮ������ | |
| C�� | Ư���к��д����ᣬ����Ư����ʹȾ�ϵ��л�ɫ����ɫ | |
| D�� | ��������ʹ�������ɫ������ɫ��Һ����ʹ�������ɫ������ɫ |
| A�� | 18O��31P��119Sn | B�� | 27Al��19F��12C | ||
| C�� | 7N��15P��33As��51Sb��83Bi | D�� | ֻ��һ�����Ӳ��ԭ�� |
| A�� | ��ϵͳ������ ������Ϊ��4��7-����-3-�һ����� ������Ϊ��4��7-����-3-�һ����� | |
| B�� | �����ʵ�����Na��NaOH��NaHCO3�ֱ����������л�� ����ַ�Ӧ�����ĸ��л�������ʵ���֮��Ϊ2��3��6����֪���Դ�С��ϵ ����ַ�Ӧ�����ĸ��л�������ʵ���֮��Ϊ2��3��6����֪���Դ�С��ϵ ��H2CO3�� ��H2CO3�� �� �� | |
| C�� | �Ҵ��Ͷ����ѻ�Ϊͬ���칹�壬CH3-CH=CH-CH3��C3H6һ����Ϊͬϵ�� | |
| D�� | �����ʡ���֬���Ǿ�����һ��������ˮ�� |
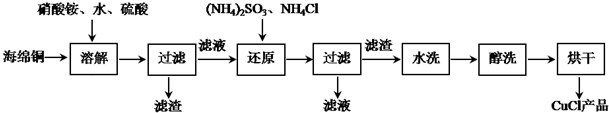
 ��
��

 ��
�� ����Ȼ�ͭ��Һ�Ļ�ѧ����ʽΪCuCl2$\frac{\underline{\;ͨ��\;}}{\;}$Cu+Cl2�����������У�Cu2+�����������ƶ���Cl-������ʧ���ӣ�����������Ӧ�����������ԭ�������������У������0.4mol�ĵ��ӷ���ת�ƣ�����������������������12.8g��
����Ȼ�ͭ��Һ�Ļ�ѧ����ʽΪCuCl2$\frac{\underline{\;ͨ��\;}}{\;}$Cu+Cl2�����������У�Cu2+�����������ƶ���Cl-������ʧ���ӣ�����������Ӧ�����������ԭ�������������У������0.4mol�ĵ��ӷ���ת�ƣ�����������������������12.8g��