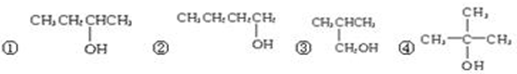
��Ŀ����
16����ͼ����ʵ���ҽ��ж��������Ʊ�����֤����ʵ������װ�ã����̶ֹ�װ��δ������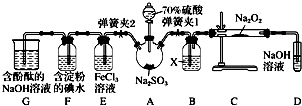
��1��װ��B���Լ�X��Ũ���ᣬװ��D��ʢ��NaOH��Һ������������δ��Ӧ��SO2����ֹ��Ⱦ������
��2���رյ��ɼ�2�����ɼ�1��ע����������û������ƿ�й��壬����SO2��Na2O2��Ӧ�Ƿ����������ɵIJ����������ǽ������ǵ�ľ������D�Թܿڴ�����ľ���Ƿ�ȼ��
��3���رյ��ɼ�1���ɼ�2�������������E��F��G�У���˵��I-��ԭ������SO2������ΪF����Һ��ɫ��ȥ��������Ӧ�����ӷ���ʽ��SO2+I2+2H2O=2I-+SO42-+4H+��
��4��Ϊ����֤E��SO2��FeCl3������������ԭ��Ӧ�����������ʵ�飺
ȡE�е���Һ������Һ�м�����ϡ�����ữ��BaCl2��Һ��������ɫ������˵��SO2��FeCl3������������ԭ��Ӧ�����������Ƿ���������������������������������ԭ����E���ܽ��SO2��ϡ���ᷴӦҲ����SO42-��
���� A���Ʊ���������Na2SO3+H2SO4��Ũ���TNa2SO4+H2O+SO2������������Ϊ�������壬X�����������ѡ��Ũ���ᣬC�м���SO2��Na2O2��Ӧ�Ƿ����������������ǵ�ľ������D�Թܿڴ�����ľ���Ƿ�ȼ��D������������Һ����ʣ��Ķ�������ֹ��Ⱦ������Eװ����֤��������Ļ�ԭ�ԣ�2FeCl3+SO2+2H2O�T2FeCl2+H2SO4+2HCl��F����I-��ԭ������SO2��SO2+I2+2H2O=2I-+SO42-+4H+��Gװ����֤��������Ϊ�������壬�����ն�������ֹ��Ⱦ������
��1���������������������ѡ��Ũ���ᣬD������������Һ����ʣ��Ķ�������
��2�����������Ĵ��ڣ��ɸ�������������ȼ�Խ��м��飻
��3������������ԭ��Ӧ�У���ԭ���Ļ�ԭ��ǿ�ڻ�ԭ�����жϣ��������ˮ������ʾ��ɫ������������ԭ�ⵥ��Ϊ������ʱ����ɫ��ȥ��
��4���������ǿ�����ԣ���������ԭ�Խ�ǿ�Ķ�������Ϊ���ᣮ
��� �⣺A���Ʊ���������X�����������C�м���SO2��Na2O2��Ӧ�Ƿ���������D������������Һ����ʣ��Ķ�������ֹ��Ⱦ������Eװ����֤��������Ļ�ԭ�ԣ�F����I-��ԭ������SO2��Gװ����֤��������Ϊ�������壬�����ն�������ֹ��Ⱦ������
��1��װ��B���Լ�X����������ʢ���Լ���Ũ���ᣬ�����������������Ʒ�Ӧ�����������ƺ�ˮ�����ӷ���ʽΪSO2+2OH-=SO32-+H2O������װ��D��ʢ��NaOH��Һ�������ǣ�����δ��Ӧ��SO2����ֹ��Ⱦ������
�ʴ�Ϊ��Ũ�������δ��Ӧ��SO2����ֹ��Ⱦ������
��2������SO2��Na2O2��Ӧ�Ƿ����������ɵķ����ǣ��������ǵ�ľ������D�Թܿڴ�����ľ���Ƿ�ȼ��
�ʴ�Ϊ���������ǵ�ľ������D�Թܿڴ�����ľ���Ƿ�ȼ��
��3��F�е����ӷ���ʽΪ��SO2+I2+2H2O=2I-+SO42-+4H+���÷�Ӧ�ж�������Ϊ��ԭ����������Ϊ��ԭ���������ԭ��Ӧ�У���ԭ���Ļ�ԭ��ǿ�ڻ�ԭ�����˵��I-��ԭ������SO2������Ϊ��F����Һ��ɫ��ȥ��
�ʴ�Ϊ��F����Һ��ɫ��ȥ�� SO2+I2+2H2O=2I-+SO42-+4H+��
��4��FeCl3��SO2���ã����������������ԣ�����������л�ԭ�ԣ��������ܽ�������������Ϊ���ᣬ��������ԭΪ�������ӣ���2FeCl3+SO2+2H2O�T2FeCl2+H2SO4+2HCl����ͨ��������������ӣ�����÷�Ӧ�ķ������������ǿ�����ԣ�����ʵ��������Ĵ��ڸ��Ÿ�SO2��FeCl3��Ӧ����֤��3SO2SO2+2HNO3+2H2O=3H2SO4+2NO������ȡE�е���Һ������Һ�м�����ϡ�����ữ��BaCl2��Һ��������ɫ��������������Ӳ�һ��������SO2��FeCl3��Ӧ����ʸ�ʵ����Ʋ�������
�ʴ�Ϊ����������E���ܽ��SO2��ϡ���ᷴӦҲ����SO42-��
���� ���⿼���˶����������ʼ����飬��Ŀ�Ѷ��еȣ�ע�����ն�������Ļ�ѧ���ʼ����鷽������ȷ���������ϢΪ�����Ĺؼ�������������ѧ���ķ�����������������ѧʵ��������

 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д�| A�� | B�� | C�� | D�� |
 |  |  |  |
| ֤��̼�ķǽ����Աȹ�ǿ | ̽��SO2��Ư���� | ����һ�����ʵ���Ũ�ȵ�ϡ���� | ��ȡ����Fe��OH��3���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| Ԫ�� | I1 | I2 | I3 | I4 |
| X | 500 | 4��600 | 6��900 | 9��500 |
| Y | 580 | 1��800 | 2��700 | 11��600 |
| A�� | Ԫ��X�ij������ϼ���+1 | |
| B�� | Ԫ��Y�Ǣ�A��Ԫ�� | |
| C�� | Ԫ��X�����γɻ�����ʱ����ѧʽ������X2O��X2O2 | |
| D�� | ��Ԫ��Y���ڵ������ڣ���������ˮ���ҷ�Ӧ |
| A�� | ��ع����ң�﮵缫��ӦʽΪLi-e-�TLi+ | |
| B�� | ����ʯī������ʱ����ػ��ܼ������� | |
| C�� | ��ع���ʱ��������е�ClO4-�˶�����ص�ʯī�� | |
| D�� | ���������˻�ԭ��Ӧ |
| A�� | X��Y�ĵ��ʾ����нϸߵ��۷е� | |
| B�� | ����������Ӧˮ�����������ǿ������˳��W��Y��X | |
| C�� | ԭ�Ӱ뾶�ɴ�С��˳��X��Y��Z | |
| D�� | Z��W�γɵĻ������мȺ������Ӽ����ֺ��й��ۼ� |
 һ���¶��£����ݻ�ΪV L���ܱ������н��з�Ӧ��aN��g��?bM��g����M��N�����ʵ�����ʱ��ı仯������ͼ��ʾ��
һ���¶��£����ݻ�ΪV L���ܱ������н��з�Ӧ��aN��g��?bM��g����M��N�����ʵ�����ʱ��ı仯������ͼ��ʾ��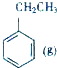 +CO2��g��?
+CO2��g��?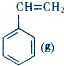 +CO��g��+H2O��g����H
+CO��g��+H2O��g����H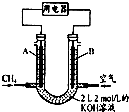
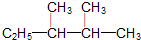 ����ϵͳ��������������2��3-�������飻
����ϵͳ��������������2��3-�������飻